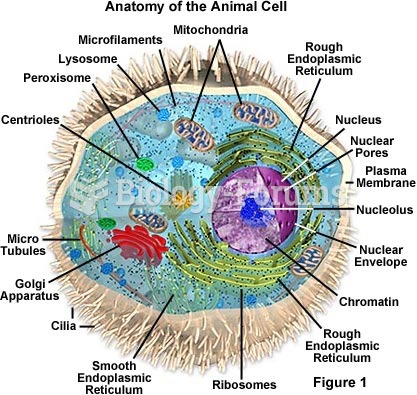
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Lower drug doses for elderly patients should be used first, with titrations of the dose as tolerated to prevent unwanted drug-related pharmacodynamic effects.
Did you know?
The most common treatment options for addiction include psychotherapy, support groups, and individual counseling.
Did you know?
Vaccines prevent between 2.5 and 4 million deaths every year.
Did you know?
Egg cells are about the size of a grain of sand. They are formed inside of a female's ovaries before she is even born.
Did you know?
The oldest recorded age was 122. Madame Jeanne Calment was born in France in 1875 and died in 1997. She was a vegetarian and loved olive oil, port wine, and chocolate.