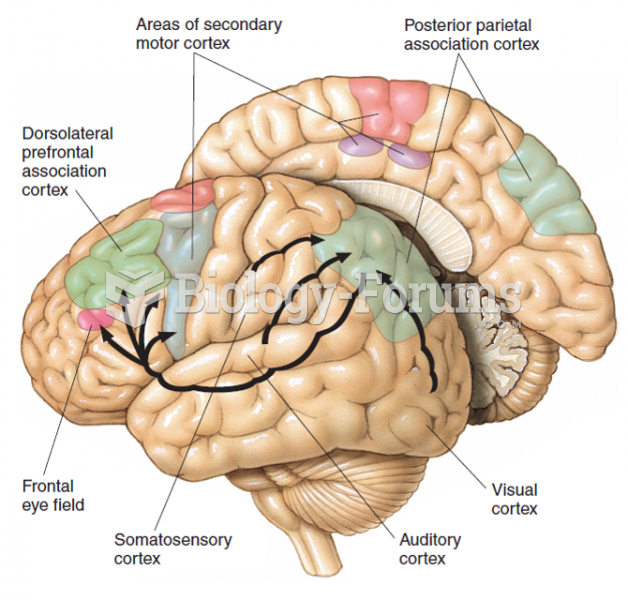
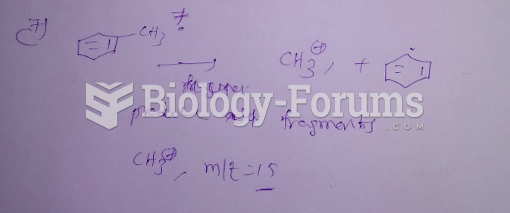|
|
|
Bacteria have flourished on the earth for over three billion years. They were the first life forms on the planet.
One way to reduce acid reflux is to lose two or three pounds. Most people lose weight in the belly area first when they increase exercise, meaning that heartburn can be reduced quickly by this method.
Bisphosphonates were first developed in the nineteenth century. They were first investigated for use in disorders of bone metabolism in the 1960s. They are now used clinically for the treatment of osteoporosis, Paget's disease, bone metastasis, multiple myeloma, and other conditions that feature bone fragility.
Parkinson's disease is both chronic and progressive. This means that it persists over a long period of time and that its symptoms grow worse over time.
More than 2,500 barbiturates have been synthesized. At the height of their popularity, about 50 were marketed for human use.
 The Land Ordinance of 1785 called for surveying and dividing the Western Territories into square mil
The Land Ordinance of 1785 called for surveying and dividing the Western Territories into square mil
 As the glass ceiling slowly cracks, women are gaining entry into the top positions of society. Shown ...
As the glass ceiling slowly cracks, women are gaining entry into the top positions of society. Shown ...