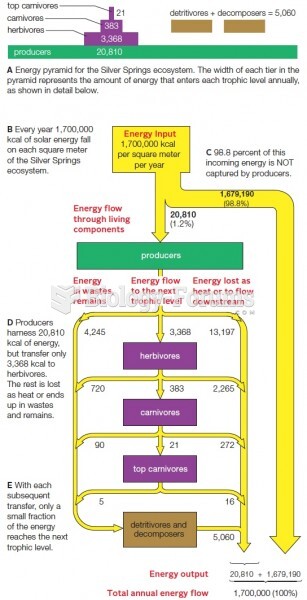
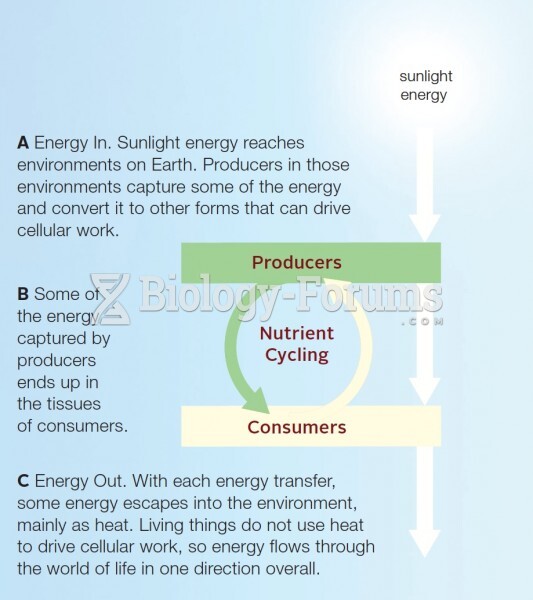
|
|
|
Certain topical medications such as clotrimazole and betamethasone are not approved for use in children younger than 12 years of age. They must be used very cautiously, as directed by a doctor, to treat any child. Children have a much greater response to topical steroid medications.
More than 150,000 Americans killed by cardiovascular disease are younger than the age of 65 years.
When blood is deoxygenated and flowing back to the heart through the veins, it is dark reddish-blue in color. Blood in the arteries that is oxygenated and flowing out to the body is bright red. Whereas arterial blood comes out in spurts, venous blood flows.
Since 1988, the CDC has reported a 99% reduction in bacterial meningitis caused by Haemophilus influenzae, due to the introduction of the vaccine against it.
Individuals are never “cured” of addictions. Instead, they learn how to manage their disease to lead healthy, balanced lives.



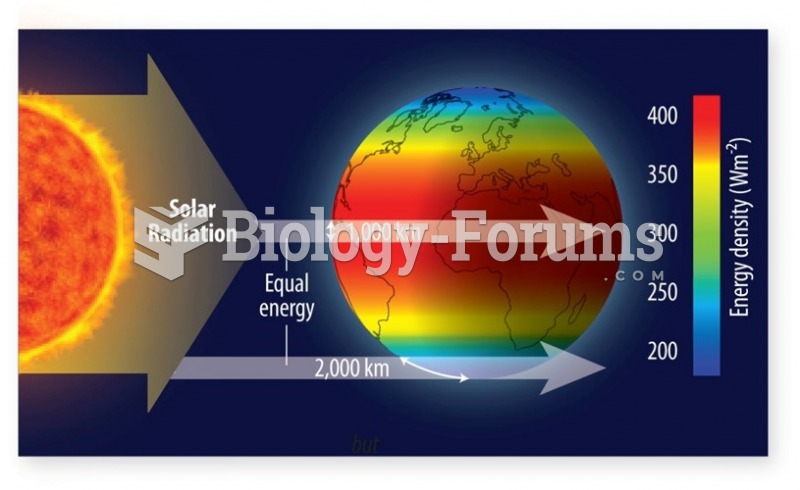
![Given the values of sin θ, determine the values of θ over the interval [0, 2pi]](https://biology-forums.com/gallery/42/medium_6_04_01_21_12_54_15.png)