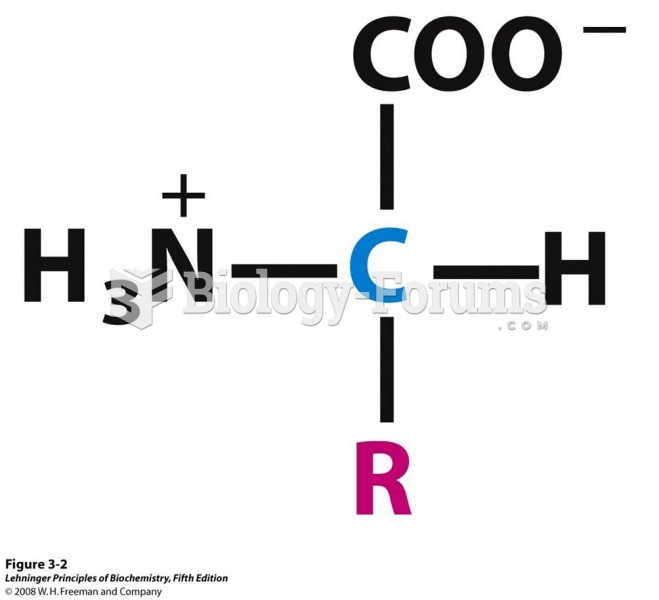
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
In ancient Rome, many of the richer people in the population had lead-induced gout. The reason for this is unclear. Lead poisoning has also been linked to madness.
Did you know?
If all the neurons in the human body were lined up, they would stretch more than 600 miles.
Did you know?
Atropine, along with scopolamine and hyoscyamine, is found in the Datura stramonium plant, which gives hallucinogenic effects and is also known as locoweed.
Did you know?
Women are 50% to 75% more likely than men to experience an adverse drug reaction.
Did you know?
Medication errors are more common among seriously ill patients than with those with minor conditions.