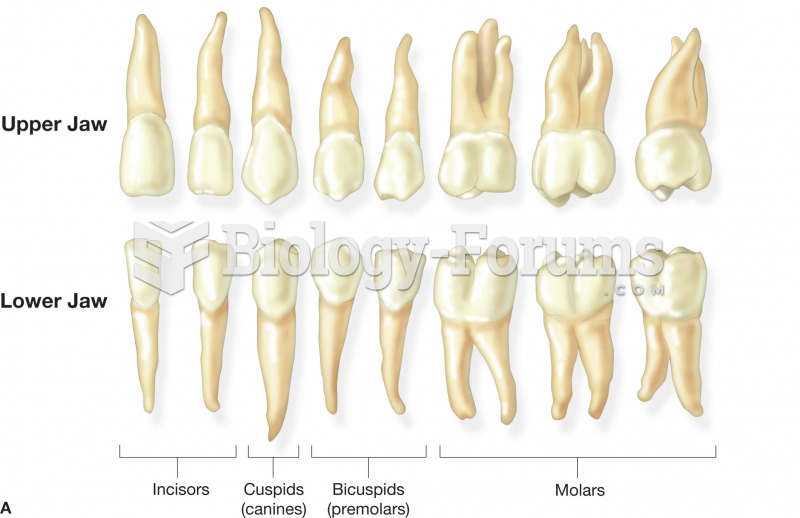
|
|
|
Patients who have undergone chemotherapy for the treatment of cancer often complain of a lack of mental focus; memory loss; and a general diminution in abilities such as multitasking, attention span, and general mental agility.
The use of salicylates dates back 2,500 years to Hippocrates's recommendation of willow bark (from which a salicylate is derived) as an aid to the pains of childbirth. However, overdosage of salicylates can harm body fluids, electrolytes, the CNS, the GI tract, the ears, the lungs, the blood, the liver, and the kidneys and cause coma or death.
This year, an estimated 1.4 million Americans will have a new or recurrent heart attack.
Bacteria have been found alive in a lake buried one half mile under ice in Antarctica.
The calories found in one piece of cherry cheesecake could light a 60-watt light bulb for 1.5 hours.