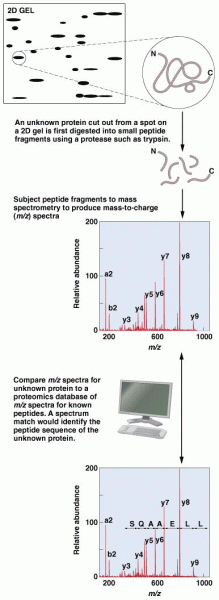
|
|
|
The toxic levels for lithium carbonate are close to the therapeutic levels. Signs of toxicity include fine hand tremor, polyuria, mild thirst, nausea, general discomfort, diarrhea, vomiting, drowsiness, muscular weakness, lack of coordination, ataxia, giddiness, tinnitus, and blurred vision.
Cucumber slices relieve headaches by tightening blood vessels, reducing blood flow to the area, and relieving pressure.
If you could remove all of your skin, it would weigh up to 5 pounds.
More than 4.4billion prescriptions were dispensed within the United States in 2016.
Certain chemicals, after ingestion, can be converted by the body into cyanide. Most of these chemicals have been removed from the market, but some old nail polish remover, solvents, and plastics manufacturing solutions can contain these substances.
 Internal structures of main sequence stars, convection zones with arrowed cycles and radiative zones
Internal structures of main sequence stars, convection zones with arrowed cycles and radiative zones
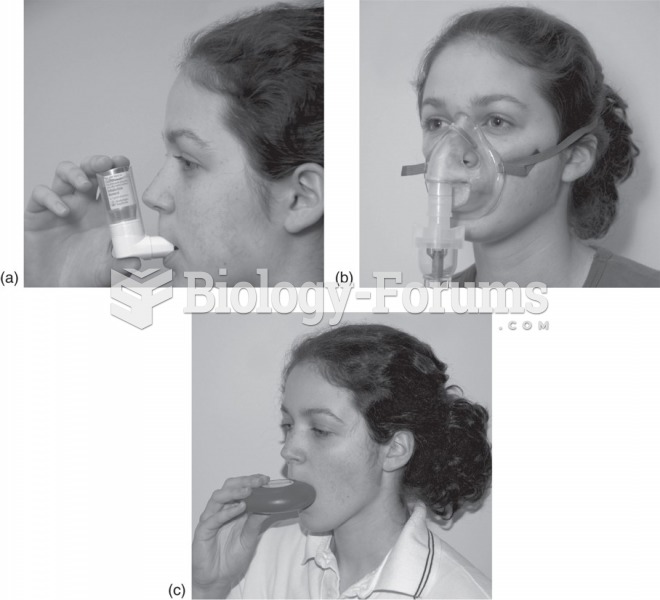 Inhalers used to deliver asthmatic drugs: (a) Metered-dose inhaler. The patient times the inhalation ...
Inhalers used to deliver asthmatic drugs: (a) Metered-dose inhaler. The patient times the inhalation ...