|
|
|
Acetaminophen (Tylenol) in overdose can seriously damage the liver. It should never be taken by people who use alcohol heavily; it can result in severe liver damage and even a condition requiring a liver transplant.
To combat osteoporosis, changes in lifestyle and diet are recommended. At-risk patients should include 1,200 to 1,500 mg of calcium daily either via dietary means or with supplements.
There are major differences in the metabolism of morphine and the illegal drug heroin. Morphine mostly produces its CNS effects through m-receptors, and at k- and d-receptors. Heroin has a slight affinity for opiate receptors. Most of its actions are due to metabolism to active metabolites (6-acetylmorphine, morphine, and morphine-6-glucuronide).
After 5 years of being diagnosed with rheumatoid arthritis, one every three patients will no longer be able to work.
Women are two-thirds more likely than men to develop irritable bowel syndrome. This may be attributable to hormonal changes related to their menstrual cycles.
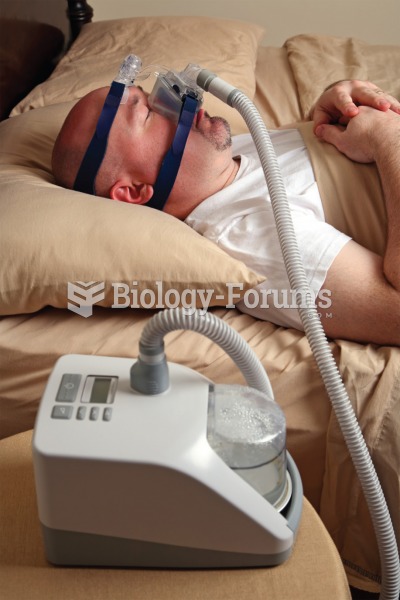 Continuous positive airway pressure (CPAP) device. The sleeping subject is receiving the benefits of
Continuous positive airway pressure (CPAP) device. The sleeping subject is receiving the benefits of
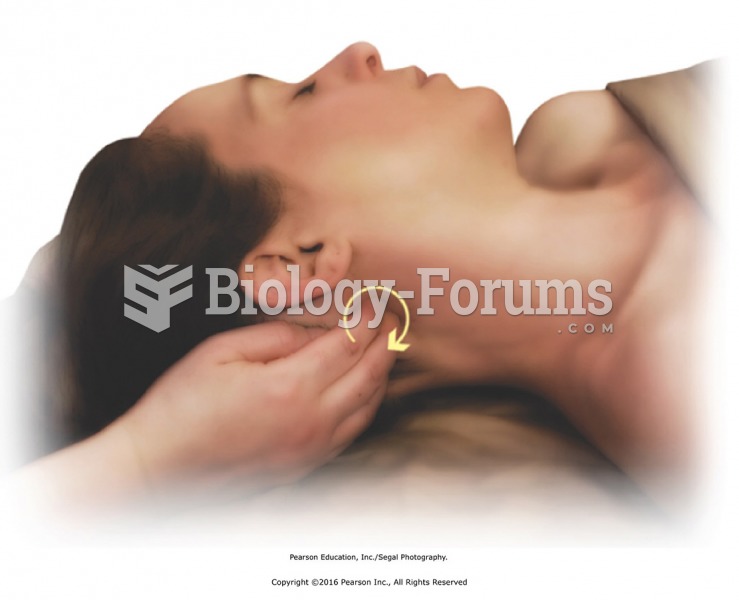
 Stacking the joints. A. Correct thumb–wrist alignment for applying direct pressure. B. Incorrect ...
Stacking the joints. A. Correct thumb–wrist alignment for applying direct pressure. B. Incorrect ...