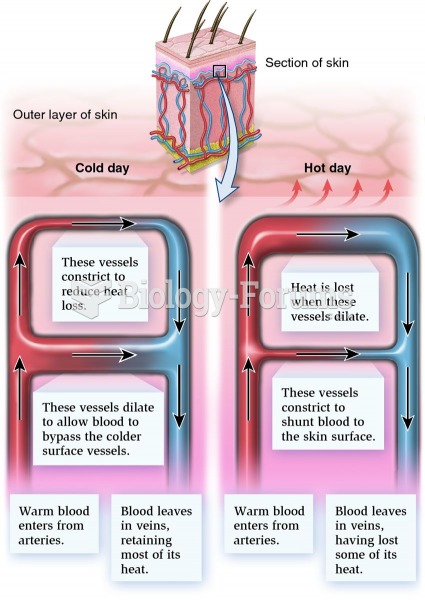
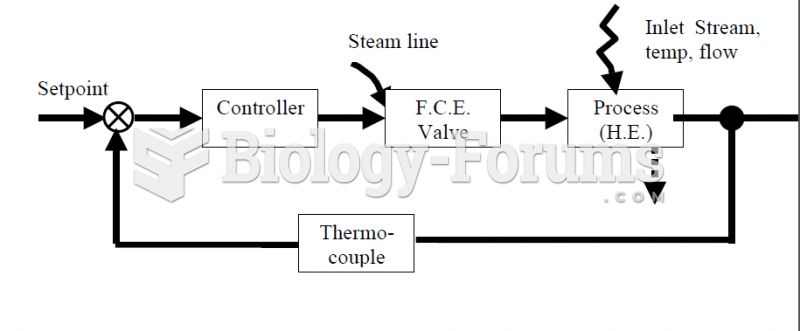
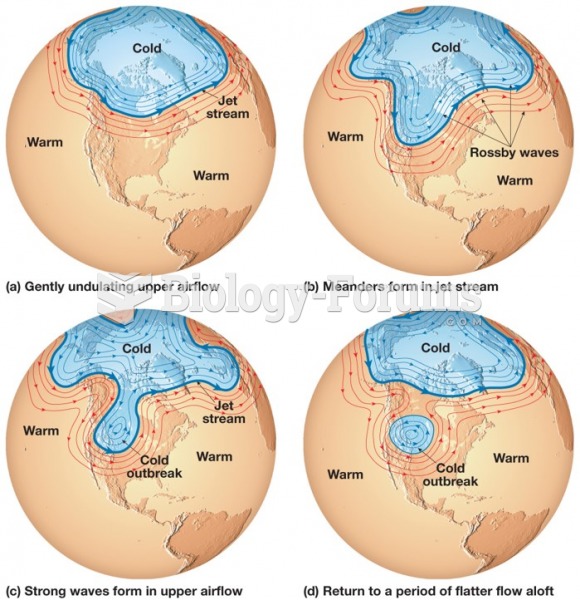
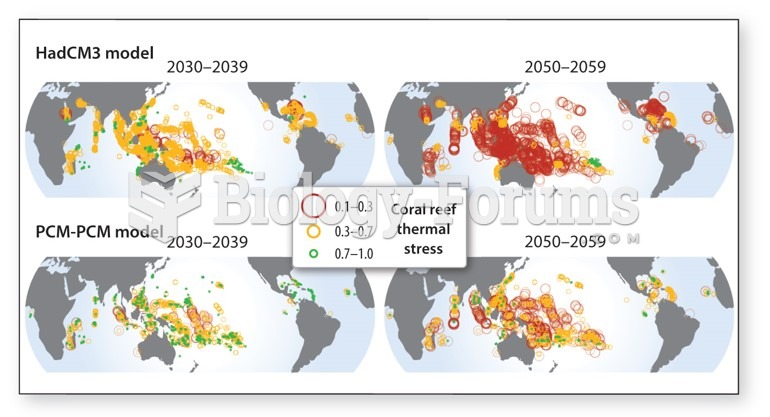
|
|
|
A headache when you wake up in the morning is indicative of sinusitis. Other symptoms of sinusitis can include fever, weakness, tiredness, a cough that may be more severe at night, and a runny nose or nasal congestion.
No drugs are available to relieve parathyroid disease. Parathyroid disease is caused by a parathyroid tumor, and it needs to be removed by surgery.
Persons who overdose with cardiac glycosides have a better chance of overall survival if they can survive the first 24 hours after the overdose.
Blood in the urine can be a sign of a kidney stone, glomerulonephritis, or other kidney problems.
More than 30% of American adults, and about 12% of children utilize health care approaches that were developed outside of conventional medicine.