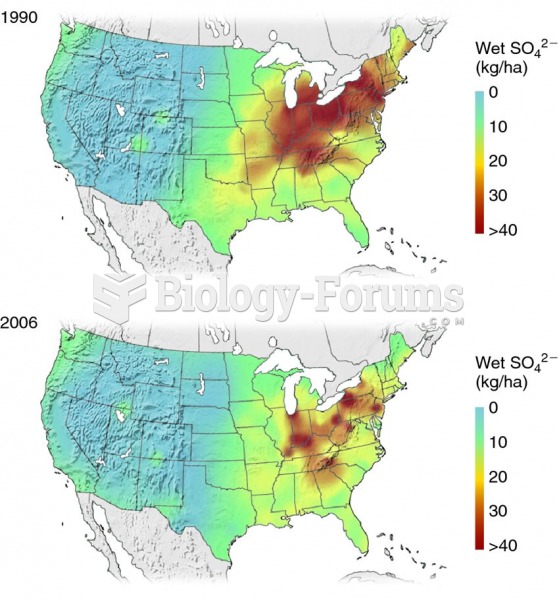
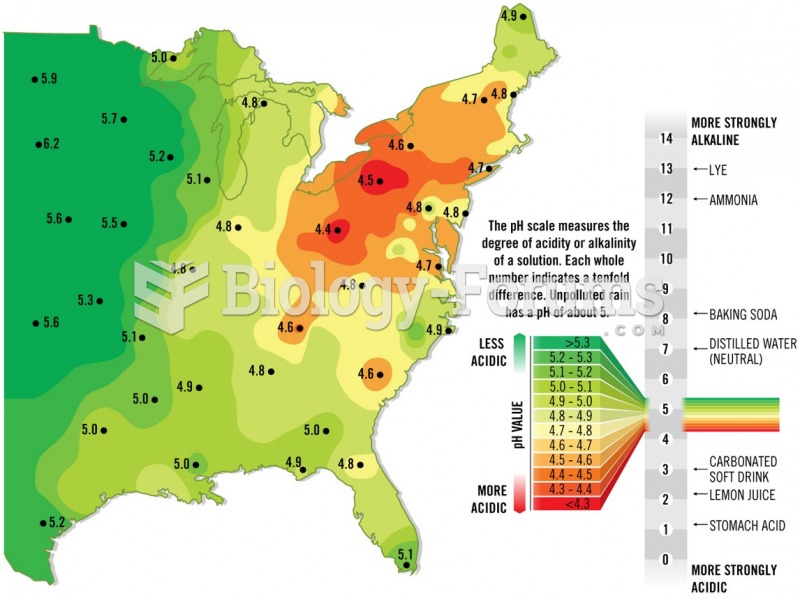
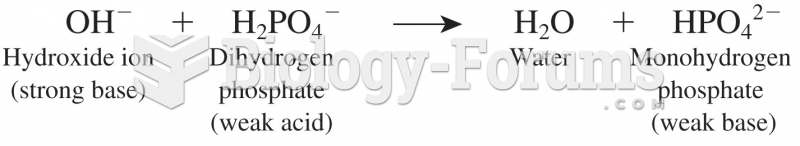
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Atropine was named after the Greek goddess Atropos, the oldest and ugliest of the three sisters known as the Fates, who controlled the destiny of men.
Did you know?
Long-term mental and physical effects from substance abuse include: paranoia, psychosis, immune deficiencies, and organ damage.
Did you know?
Medication errors are three times higher among children and infants than with adults.
Did you know?
Women are two-thirds more likely than men to develop irritable bowel syndrome. This may be attributable to hormonal changes related to their menstrual cycles.
Did you know?
On average, the stomach produces 2 L of hydrochloric acid per day.