This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
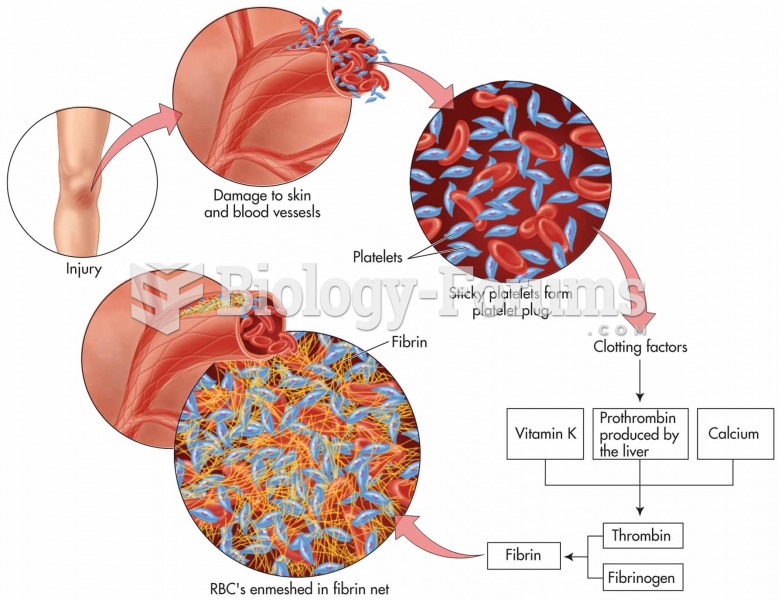
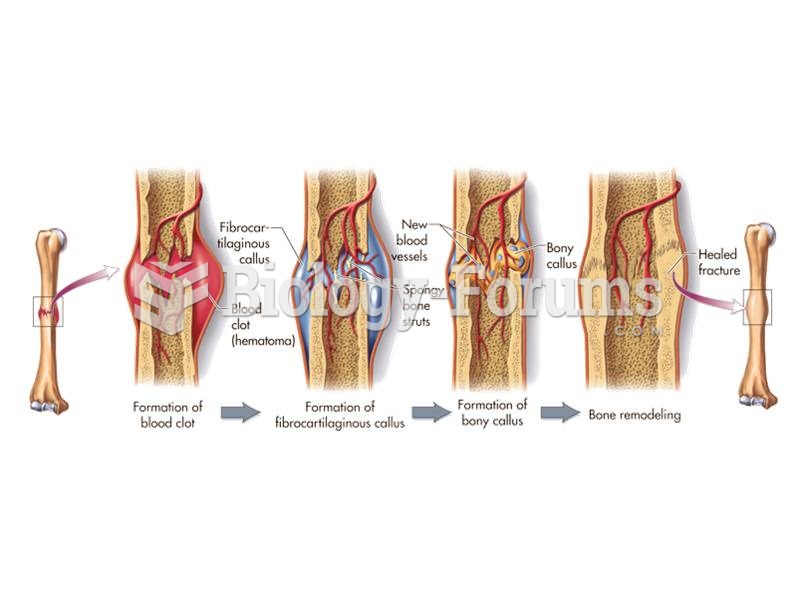
Increased intake of vitamin D has been shown to reduce fractures up to 25% in older people.
Did you know?
After a vasectomy, it takes about 12 ejaculations to clear out sperm that were already beyond the blocked area.
Did you know?
People with high total cholesterol have about two times the risk for heart disease as people with ideal levels.
Did you know?
About 3.2 billion people, nearly half the world population, are at risk for malaria. In 2015, there are about 214 million malaria cases and an estimated 438,000 malaria deaths.
Did you know?
Individuals are never “cured” of addictions. Instead, they learn how to manage their disease to lead healthy, balanced lives.