|
|
|
The average office desk has 400 times more bacteria on it than a toilet.
Hypertension is a silent killer because it is deadly and has no significant early symptoms. The danger from hypertension is the extra load on the heart, which can lead to hypertensive heart disease and kidney damage. This occurs without any major symptoms until the high blood pressure becomes extreme. Regular blood pressure checks are an important method of catching hypertension before it can kill you.
According to the CDC, approximately 31.7% of the U.S. population has high low-density lipoprotein (LDL) or "bad cholesterol" levels.
The strongest synthetic topical retinoid drug available, tazarotene, is used to treat sun-damaged skin, acne, and psoriasis.
Eat fiber! A diet high in fiber can help lower cholesterol levels by as much as 10%.
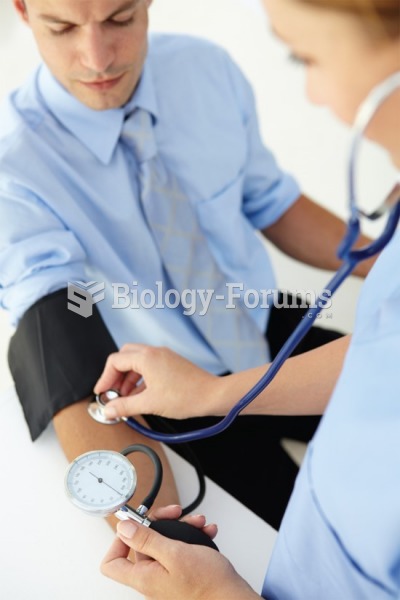 Taking a blood pressure is one of the procedures the medical assistant extern may perform under the ...
Taking a blood pressure is one of the procedures the medical assistant extern may perform under the ...
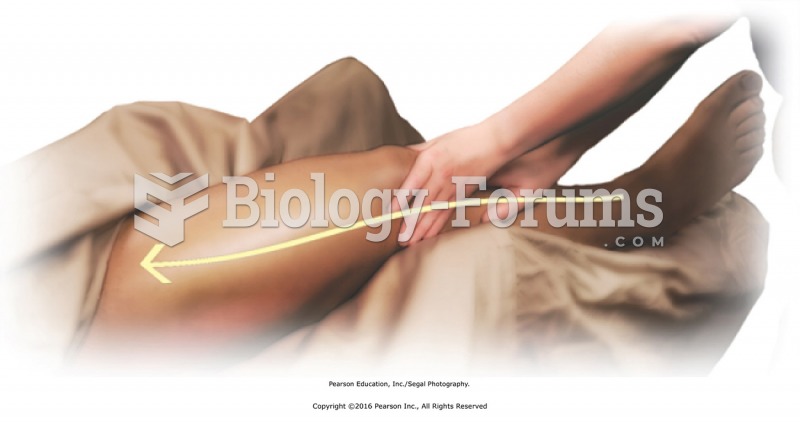 Apply lubricant to the entire right limb using basic sliding effleurage. Apply moderate pressure ...
Apply lubricant to the entire right limb using basic sliding effleurage. Apply moderate pressure ...