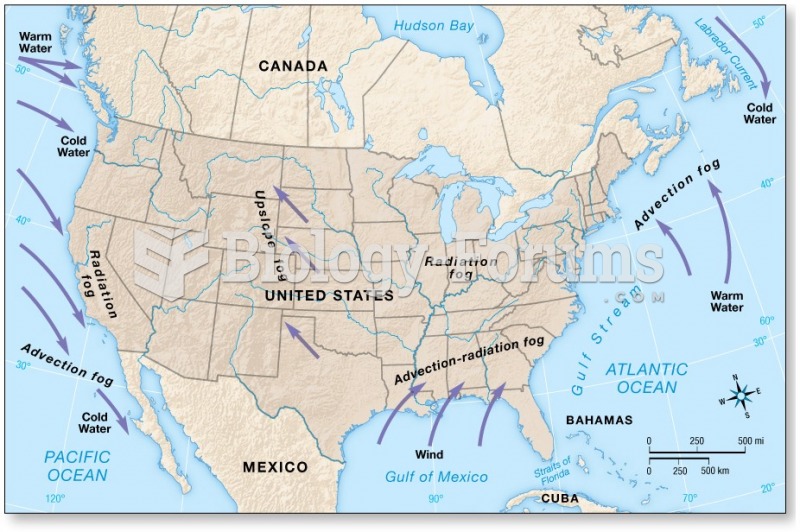
|
|
|
The average older adult in the United States takes five prescription drugs per day. Half of these drugs contain a sedative. Alcohol should therefore be avoided by most senior citizens because of the dangerous interactions between alcohol and sedatives.
Hip fractures are the most serious consequences of osteoporosis. The incidence of hip fractures increases with each decade among patients in their 60s to patients in their 90s for both women and men of all populations. Men and women older than 80 years of age show the highest incidence of hip fractures.
There are 20 feet of blood vessels in each square inch of human skin.
About 100 new prescription or over-the-counter drugs come into the U.S. market every year.
According to the American College of Allergy, Asthma & Immunology, more than 50 million Americans have some kind of food allergy. Food allergies affect between 4 and 6% of children, and 4% of adults, according to the CDC. The most common food allergies include shellfish, peanuts, walnuts, fish, eggs, milk, and soy.
 The Kingsley plantation, on Fort George Island in Jacksonville, Florida. Zephaniah Kingsley, the own
The Kingsley plantation, on Fort George Island in Jacksonville, Florida. Zephaniah Kingsley, the own
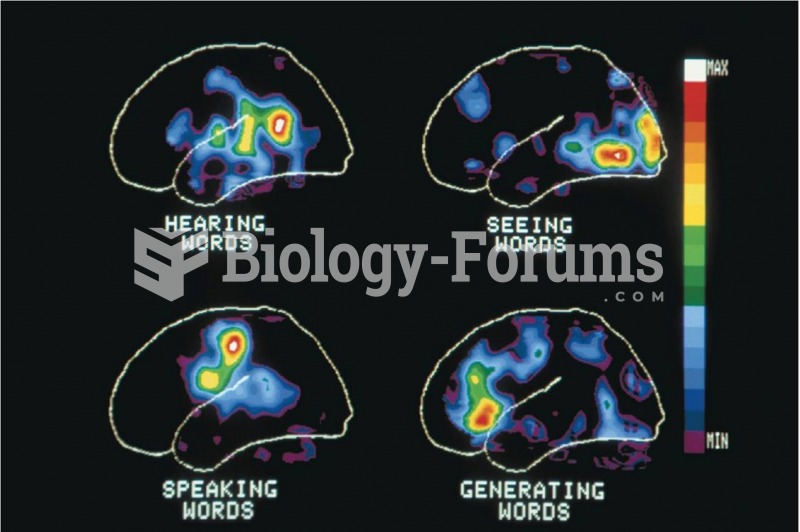 Is the self the same as the brain? Materialists contend that in the final analysis, mental states ...
Is the self the same as the brain? Materialists contend that in the final analysis, mental states ...