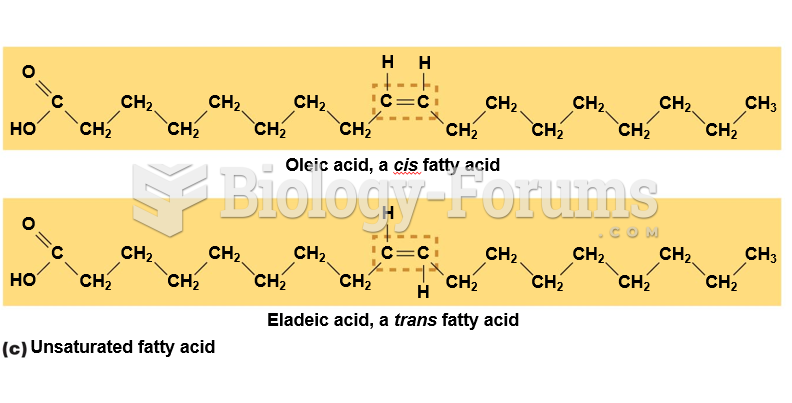
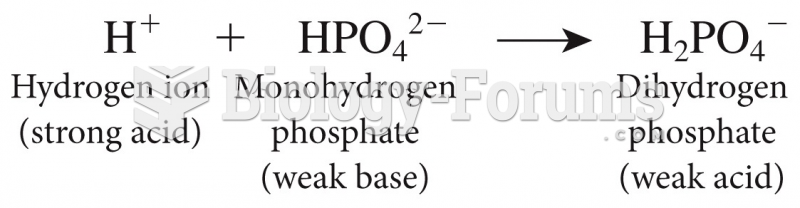This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
The first oral chemotherapy drug for colon cancer was approved by FDA in 2001.
Did you know?
Cyanide works by making the human body unable to use oxygen.
Did you know?
Blood in the urine can be a sign of a kidney stone, glomerulonephritis, or other kidney problems.
Did you know?
Studies show that systolic blood pressure can be significantly lowered by taking statins. In fact, the higher the patient's baseline blood pressure, the greater the effect of statins on his or her blood pressure.
Did you know?
Signs and symptoms of a drug overdose include losing consciousness, fever or sweating, breathing problems, abnormal pulse, and changes in skin color.