|
|
|
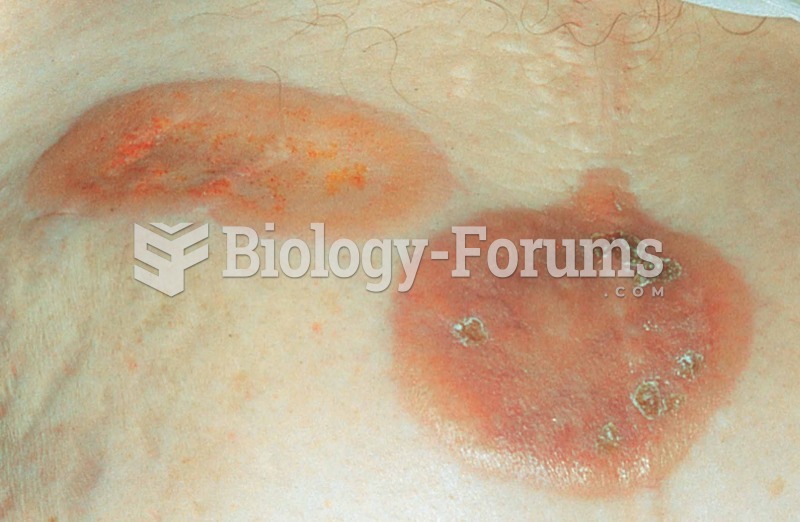
Amoebae are the simplest type of protozoans, and are characterized by a feeding and dividing trophozoite stage that moves by temporary extensions called pseudopodia or false feet.
The liver is the only organ that has the ability to regenerate itself after certain types of damage. As much as 25% of the liver can be removed, and it will still regenerate back to its original shape and size. However, the liver cannot regenerate after severe damage caused by alcohol.
It is difficult to obtain enough calcium without consuming milk or other dairy foods.
More than 2,500 barbiturates have been synthesized. At the height of their popularity, about 50 were marketed for human use.
More than nineteen million Americans carry the factor V gene that causes blood clots, pulmonary embolism, and heart disease.