|
|
|
The human body's pharmacokinetics are quite varied. Our hair holds onto drugs longer than our urine, blood, or saliva. For example, alcohol can be detected in the hair for up to 90 days after it was consumed. The same is true for marijuana, cocaine, ecstasy, heroin, methamphetamine, and nicotine.
Multiple sclerosis is a condition wherein the body's nervous system is weakened by an autoimmune reaction that attacks the myelin sheaths of neurons.
Interferon was scarce and expensive until 1980, when the interferon gene was inserted into bacteria using recombinant DNA technology, allowing for mass cultivation and purification from bacterial cultures.
Though “Krazy Glue” or “Super Glue” has the ability to seal small wounds, it is not recommended for this purpose since it contains many substances that should not enter the body through the skin, and may be harmful.
In the United States, there is a birth every 8 seconds, according to the U.S. Census Bureau's Population Clock.
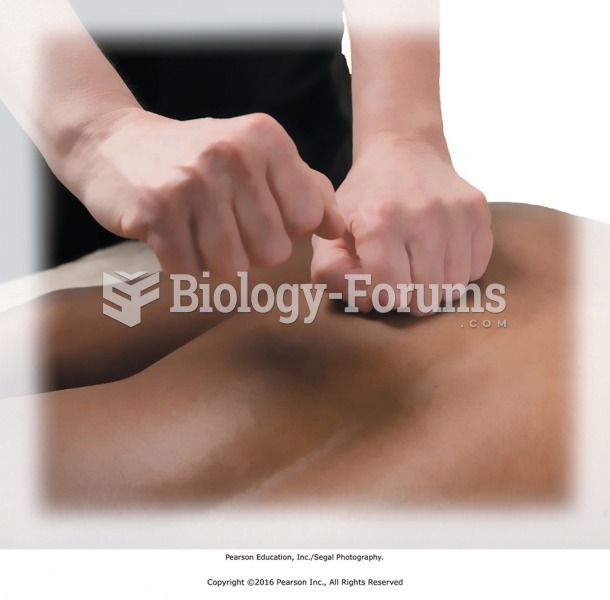 Rapping. Tapotement performed with the knuckles of a loosely closed fist, as if rapping lightly on a ...
Rapping. Tapotement performed with the knuckles of a loosely closed fist, as if rapping lightly on a ...
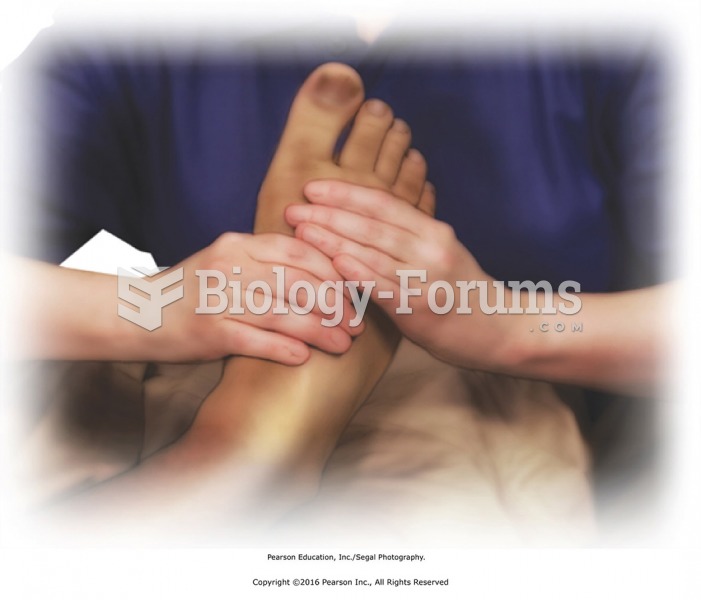 Squeeze the foot lightly from heel to toes. Fingers of hands on top with thumbs around the sides and ...
Squeeze the foot lightly from heel to toes. Fingers of hands on top with thumbs around the sides and ...