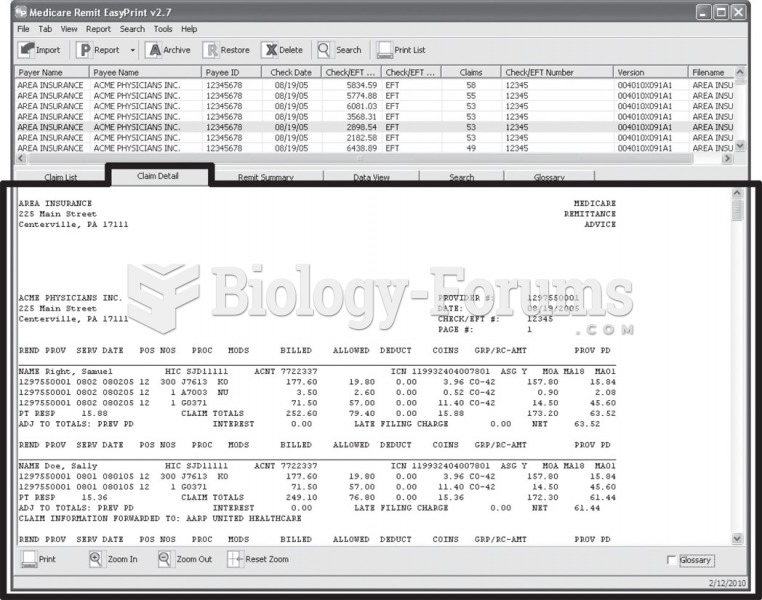
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Drug-induced pharmacodynamic effects manifested in older adults include drug-induced renal toxicity, which can be a major factor when these adults are experiencing other kidney problems.
Did you know?
According to the CDC, approximately 31.7% of the U.S. population has high low-density lipoprotein (LDL) or "bad cholesterol" levels.
Did you know?
Sperm cells are so tiny that 400 to 500 million (400,000,000–500,000,000) of them fit onto 1 tsp.
Did you know?
Stroke kills people from all ethnic backgrounds, but the people at highest risk for fatal strokes are: black men, black women, Asian men, white men, and white women.
Did you know?
Cucumber slices relieve headaches by tightening blood vessels, reducing blood flow to the area, and relieving pressure.