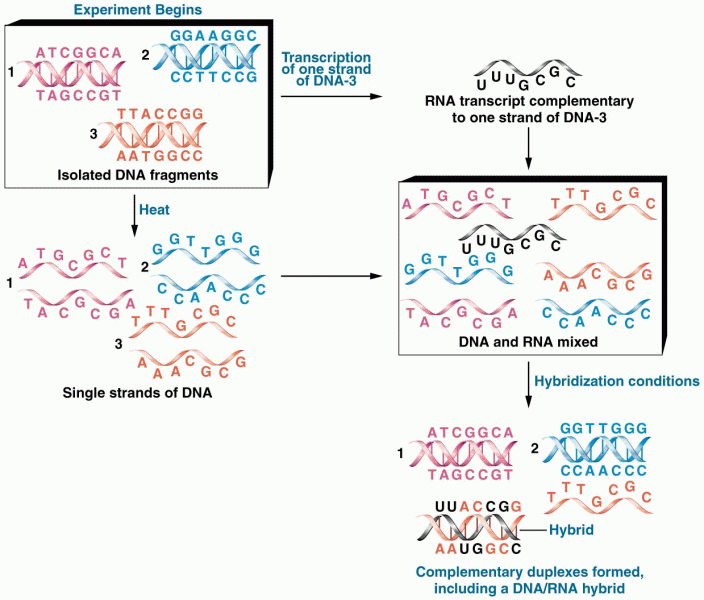
|
|
|
Patients who have been on total parenteral nutrition for more than a few days may need to have foods gradually reintroduced to give the digestive tract time to start working again.
In 1844, Charles Goodyear obtained the first patent for a rubber condom.
In 2006, a generic antinausea drug named ondansetron was approved. It is used to stop nausea and vomiting associated with surgery, chemotherapy, and radiation therapy.
Vampire bats have a natural anticoagulant in their saliva that permits continuous bleeding after they painlessly open a wound with their incisors. This capillary blood does not cause any significant blood loss to their victims.
Vital signs (blood pressure, temperature, pulse rate, respiration rate) should be taken before any drug administration. Patients should be informed not to use tobacco or caffeine at least 30 minutes before their appointment.
 Successful agriculturalists must have a basic understanding of landscape structure and process. Thes
Successful agriculturalists must have a basic understanding of landscape structure and process. Thes
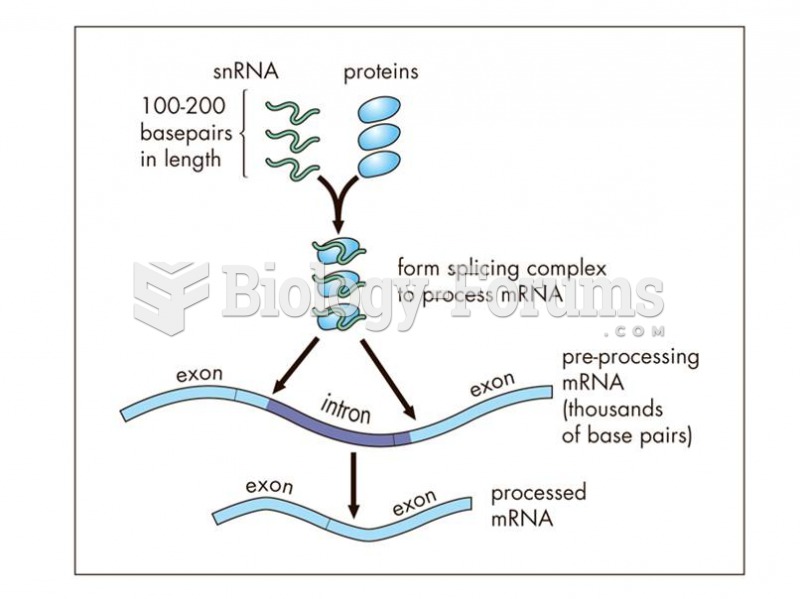 Small nuclear RNAs (snRNA), 100-200 bp in length, form part of the splicing mechanisms to process mR
Small nuclear RNAs (snRNA), 100-200 bp in length, form part of the splicing mechanisms to process mR