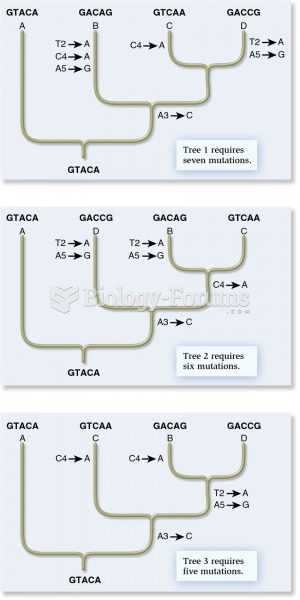
|
|
|
IgA antibodies protect body surfaces exposed to outside foreign substances. IgG antibodies are found in all body fluids. IgM antibodies are the first type of antibody made in response to an infection. IgE antibody levels are often high in people with allergies. IgD antibodies are found in tissues lining the abdomen and chest.
Blood is approximately twice as thick as water because of the cells and other components found in it.
Human stomach acid is strong enough to dissolve small pieces of metal such as razor blades or staples.
Children of people with alcoholism are more inclined to drink alcohol or use hard drugs. In fact, they are 400 times more likely to use hard drugs than those who do not have a family history of alcohol addiction.
If you could remove all of your skin, it would weigh up to 5 pounds.