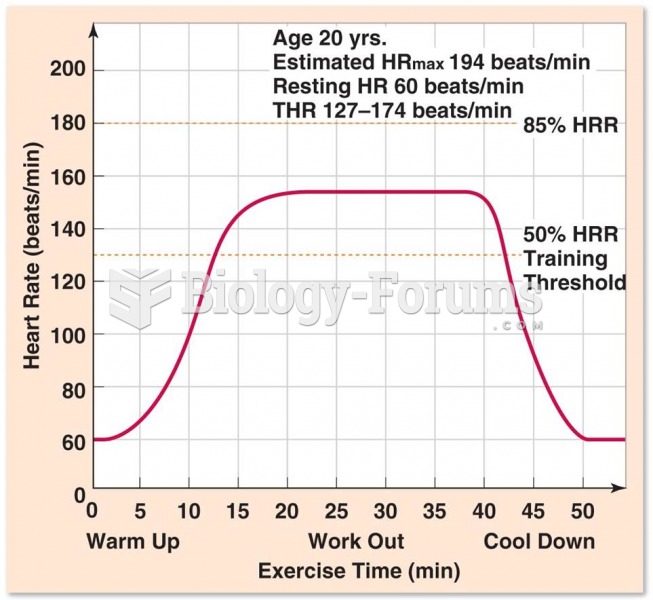
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Between 1999 and 2012, American adults with high total cholesterol decreased from 18.3% to 12.9%
Did you know?
The top 10 most important tips that will help you grow old gracefully include (1) quit smoking, (2) keep your weight down, (3) take supplements, (4) skip a meal each day or fast 1 day per week, (5) get a pet, (6) get medical help for chronic pain, (7) walk regularly, (8) reduce arguments, (9) put live plants in your living space, and (10) do some weight training.
Did you know?
Medication errors are more common among seriously ill patients than with those with minor conditions.
Did you know?
There are 60,000 miles of blood vessels in every adult human.
Did you know?
The B-complex vitamins and vitamin C are not stored in the body and must be replaced each day.