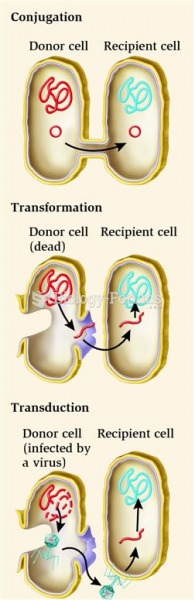
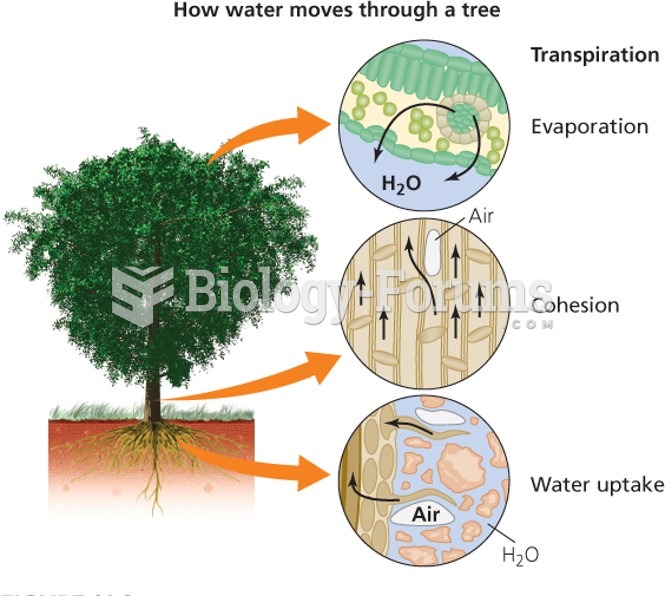
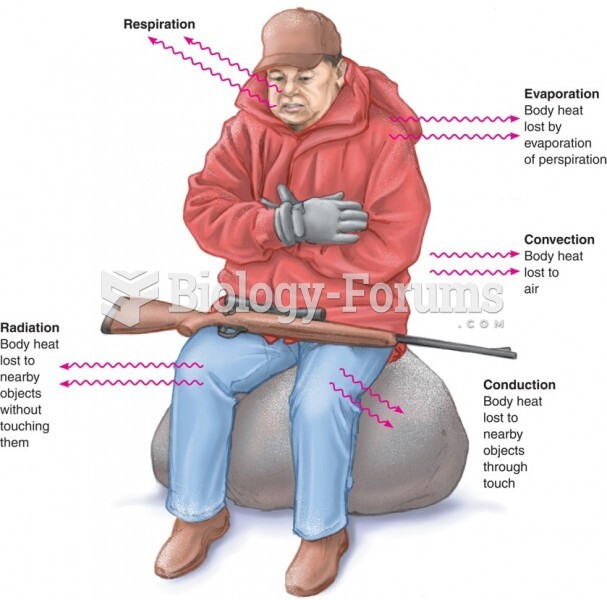
|
|
|
After a vasectomy, it takes about 12 ejaculations to clear out sperm that were already beyond the blocked area.
Inotropic therapy does not have a role in the treatment of most heart failure patients. These drugs can make patients feel and function better but usually do not lengthen the predicted length of their lives.
The most destructive flu epidemic of all times in recorded history occurred in 1918, with approximately 20 million deaths worldwide.
Nearly all drugs pass into human breast milk. How often a drug is taken influences the amount of drug that will pass into the milk. Medications taken 30 to 60 minutes before breastfeeding are likely to be at peak blood levels when the baby is nursing.
Symptoms of kidney problems include a loss of appetite, back pain (which may be sudden and intense), chills, abdominal pain, fluid retention, nausea, the urge to urinate, vomiting, and fever.