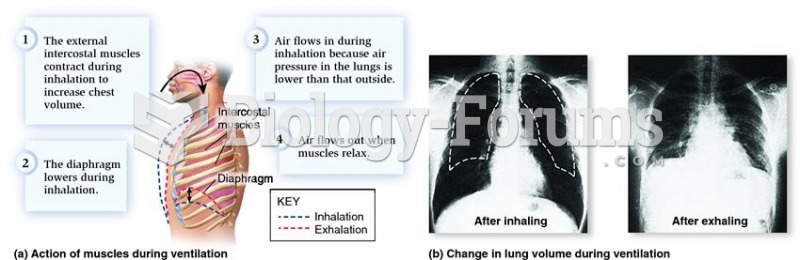
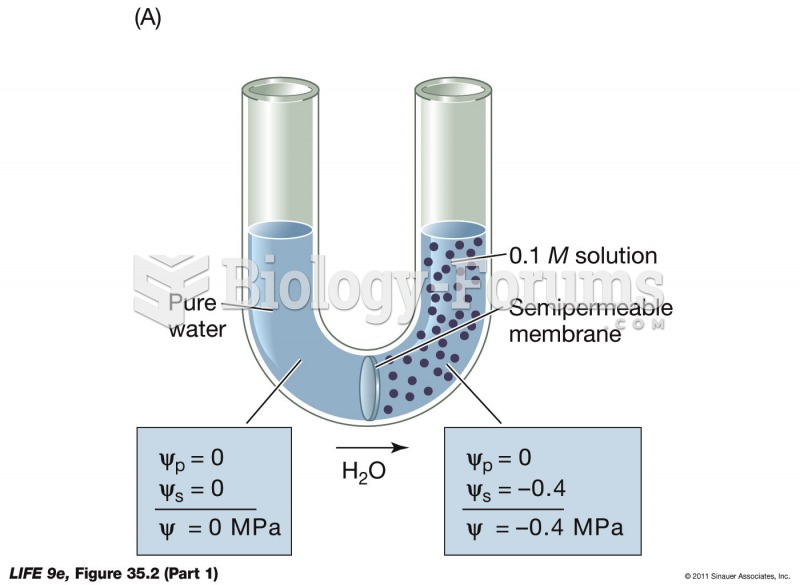
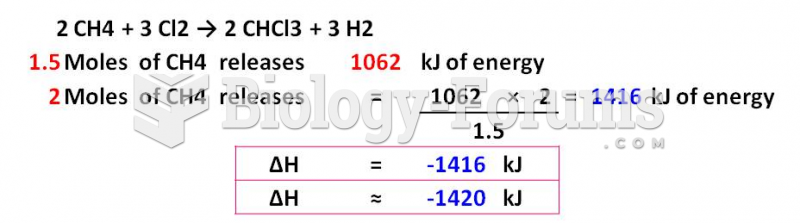
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Acetaminophen (Tylenol) in overdose can seriously damage the liver. It should never be taken by people who use alcohol heavily; it can result in severe liver damage and even a condition requiring a liver transplant.
Did you know?
The familiar sounds of your heart are made by the heart's valves as they open and close.
Did you know?
It is difficult to obtain enough calcium without consuming milk or other dairy foods.
Did you know?
The average office desk has 400 times more bacteria on it than a toilet.
Did you know?
Many of the drugs used by neuroscientists are derived from toxic plants and venomous animals (such as snakes, spiders, snails, and puffer fish).