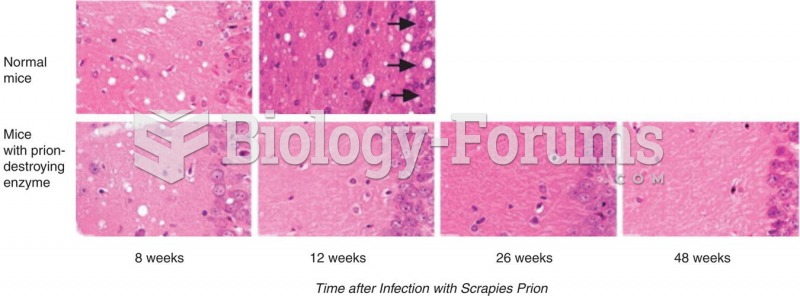
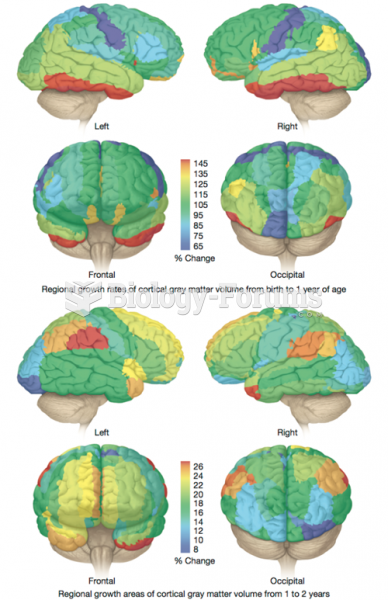
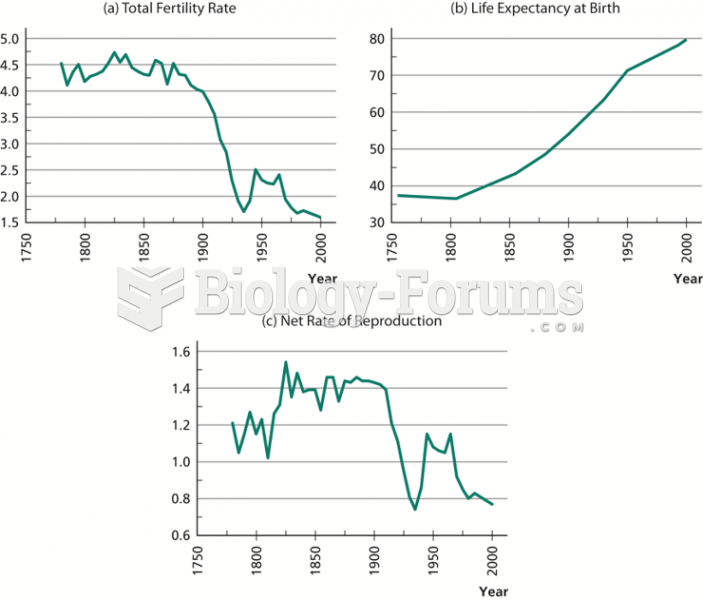
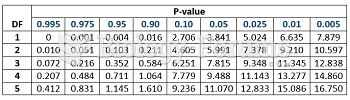
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Aspirin may benefit 11 different cancers, including those of the colon, pancreas, lungs, prostate, breasts, and leukemia.
Did you know?
Excessive alcohol use costs the country approximately $235 billion every year.
Did you know?
In 2006, a generic antinausea drug named ondansetron was approved. It is used to stop nausea and vomiting associated with surgery, chemotherapy, and radiation therapy.
Did you know?
Addicts to opiates often avoid treatment because they are afraid of withdrawal. Though unpleasant, with proper management, withdrawal is rarely fatal and passes relatively quickly.
Did you know?
There are approximately 3 million unintended pregnancies in the United States each year.