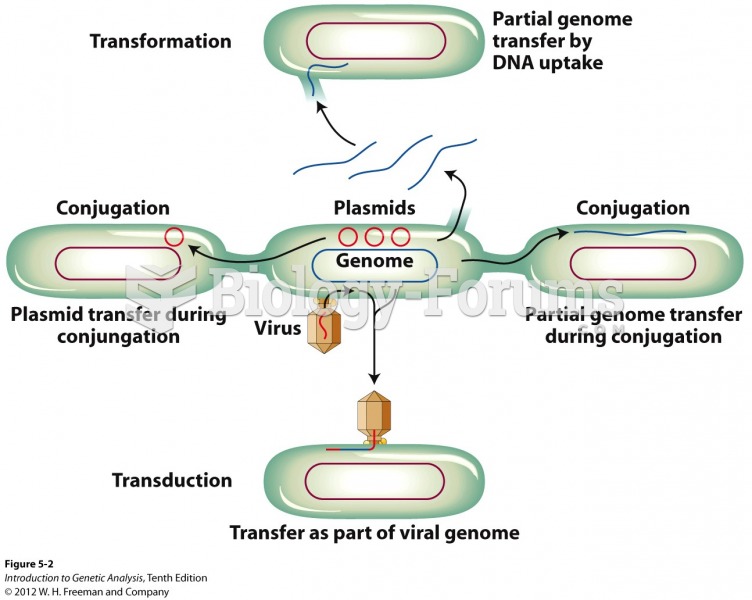
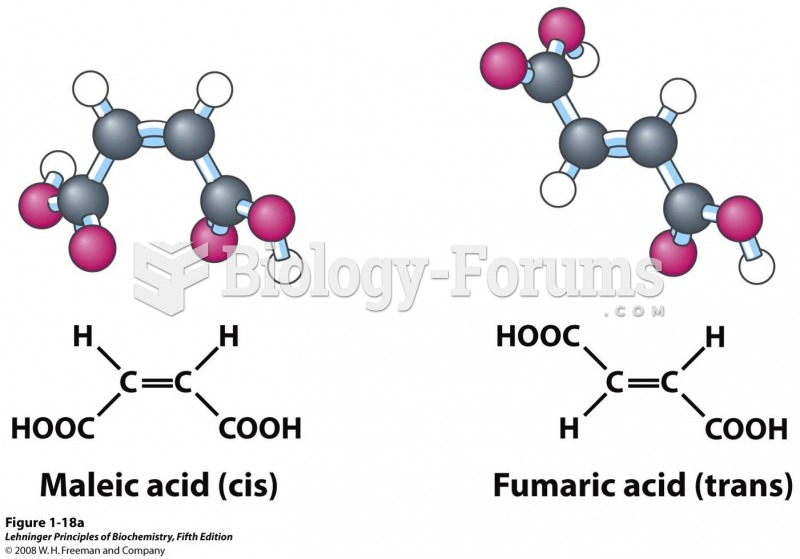
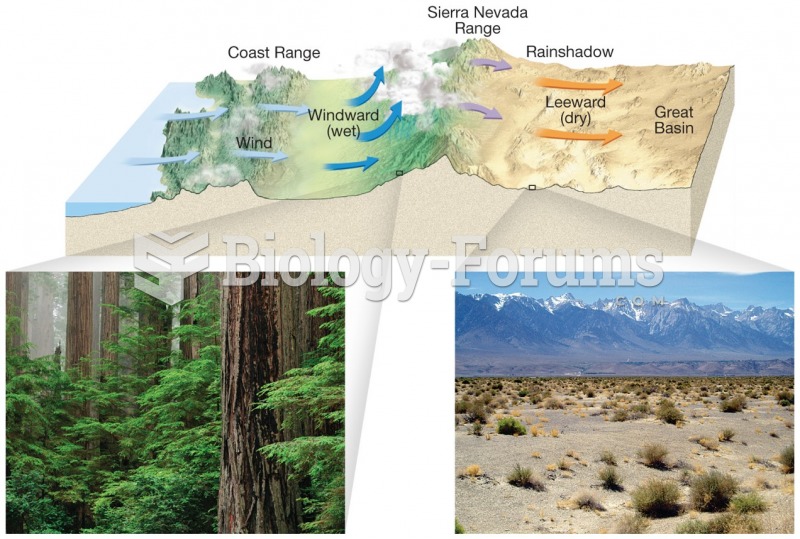
|
|
|
Increased intake of vitamin D has been shown to reduce fractures up to 25% in older people.
A seasonal flu vaccine is the best way to reduce the chances you will get seasonal influenza and spread it to others.
Though Candida and Aspergillus species are the most common fungal pathogens causing invasive fungal disease in the immunocompromised, infections due to previously uncommon hyaline and dematiaceous filamentous fungi are occurring more often today. Rare fungal infections, once accurately diagnosed, may require surgical debridement, immunotherapy, and newer antifungals used singly or in combination with older antifungals, on a case-by-case basis.
Medications that are definitely not safe to take when breastfeeding include radioactive drugs, antimetabolites, some cancer (chemotherapy) agents, bromocriptine, ergotamine, methotrexate, and cyclosporine.
In 1844, Charles Goodyear obtained the first patent for a rubber condom.