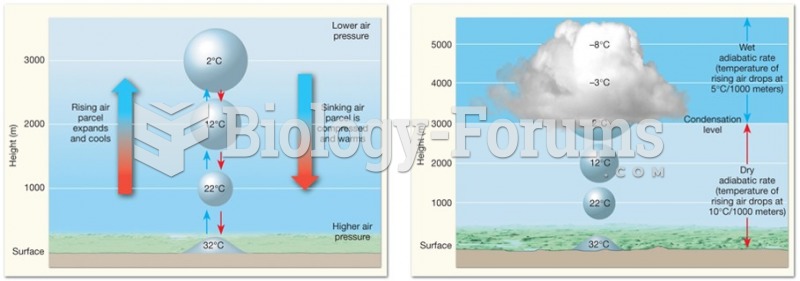
|
|
|
As many as 20% of Americans have been infected by the fungus known as Histoplasmosis. While most people are asymptomatic or only have slight symptoms, infection can progress to a rapid and potentially fatal superinfection.
A headache when you wake up in the morning is indicative of sinusitis. Other symptoms of sinusitis can include fever, weakness, tiredness, a cough that may be more severe at night, and a runny nose or nasal congestion.
On average, someone in the United States has a stroke about every 40 seconds. This is about 795,000 people per year.
Recent studies have shown that the number of medication errors increases in relation to the number of orders that are verified per pharmacist, per work shift.
There are 20 feet of blood vessels in each square inch of human skin.