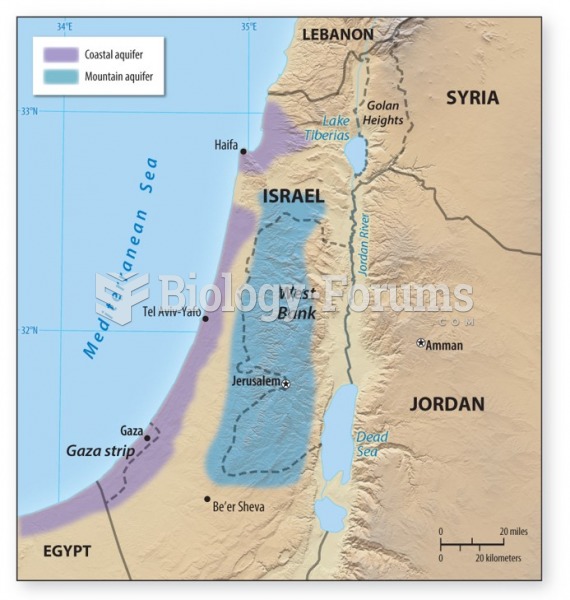
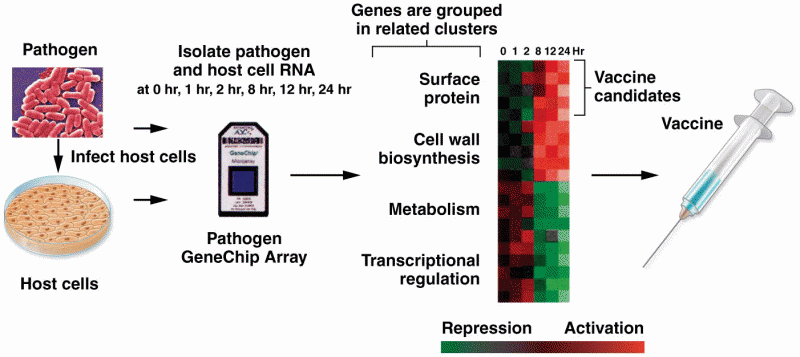
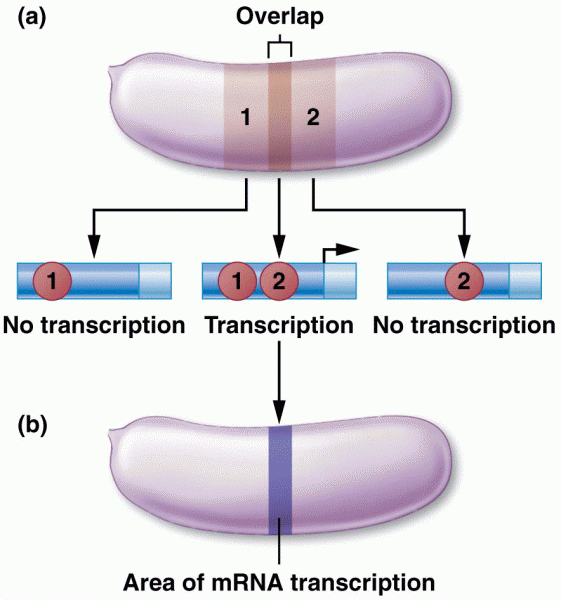
|
|
|
A seasonal flu vaccine is the best way to reduce the chances you will get seasonal influenza and spread it to others.
Bacteria have flourished on the earth for over three billion years. They were the first life forms on the planet.
Medication errors are more common among seriously ill patients than with those with minor conditions.
Critical care patients are twice as likely to receive the wrong medication. Of these errors, 20% are life-threatening, and 42% require additional life-sustaining treatments.
There used to be a metric calendar, as well as metric clocks. The metric calendar, or "French Republican Calendar" divided the year into 12 months, but each month was divided into three 10-day weeks. Each day had 10 decimal hours. Each hour had 100 decimal minutes. Due to lack of popularity, the metric clocks and calendars were ended in 1795, three years after they had been first marketed.