|
|
|
The eye muscles are the most active muscles in the whole body. The external muscles that move the eyes are the strongest muscles in the human body for the job they have to do. They are 100 times more powerful than they need to be.
Many medications that are used to treat infertility are injected subcutaneously. This is easy to do using the anterior abdomen as the site of injection but avoiding the area directly around the belly button.
Less than one of every three adults with high LDL cholesterol has the condition under control. Only 48.1% with the condition are being treated for it.
The calories found in one piece of cherry cheesecake could light a 60-watt light bulb for 1.5 hours.
After a vasectomy, it takes about 12 ejaculations to clear out sperm that were already beyond the blocked area.
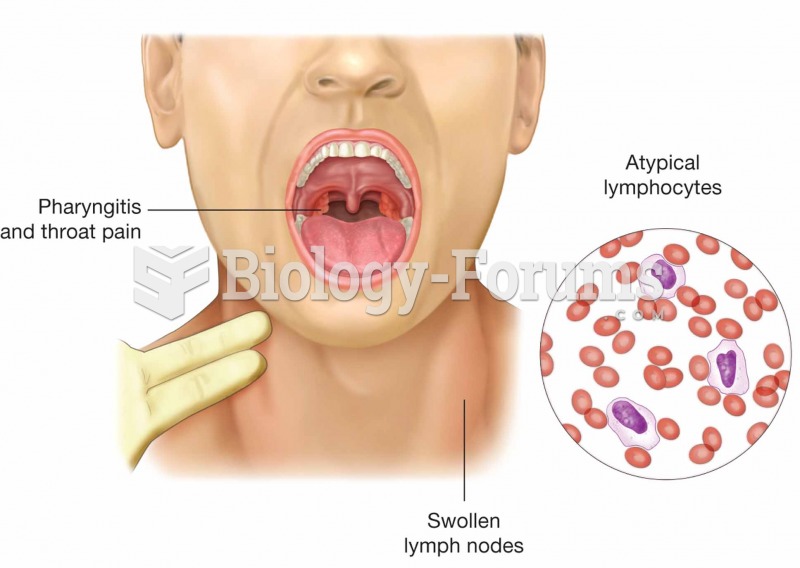 Mononucleosis is caused by the Epstein–Barr virus. Symptoms of the infectious disease are swollen pa
Mononucleosis is caused by the Epstein–Barr virus. Symptoms of the infectious disease are swollen pa
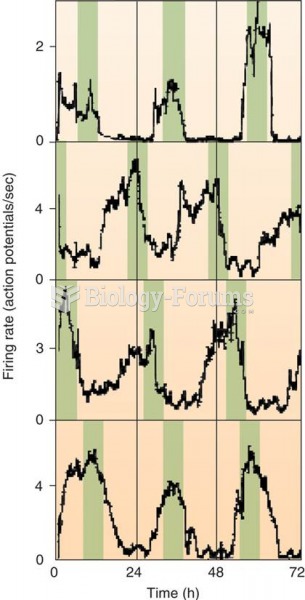 Firing Rate of Individual SCN Neurons in a Tissue Culture Color bars have been added to emphasize th
Firing Rate of Individual SCN Neurons in a Tissue Culture Color bars have been added to emphasize th