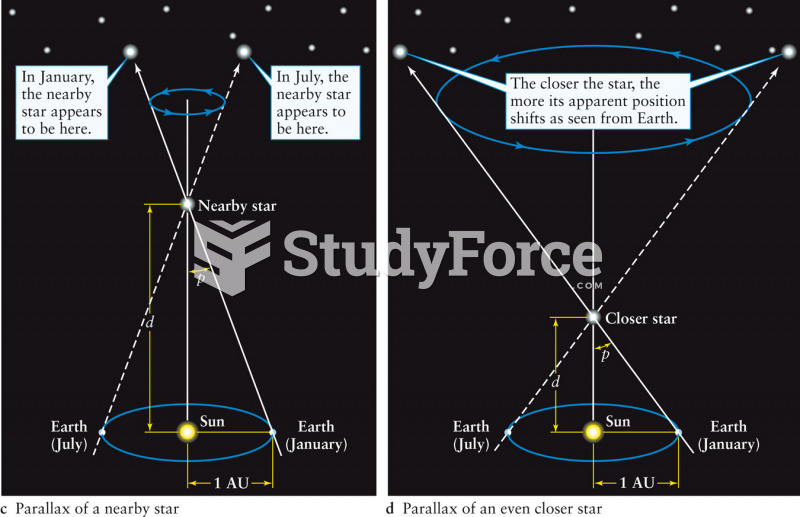
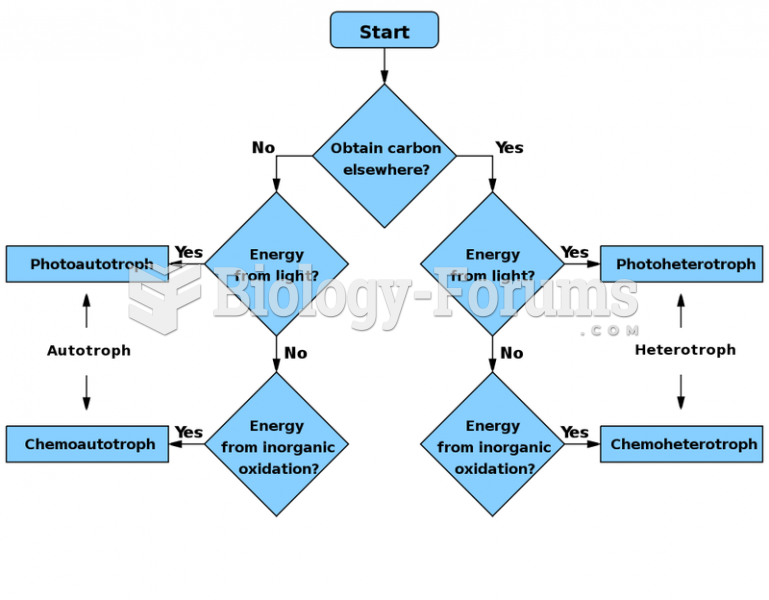
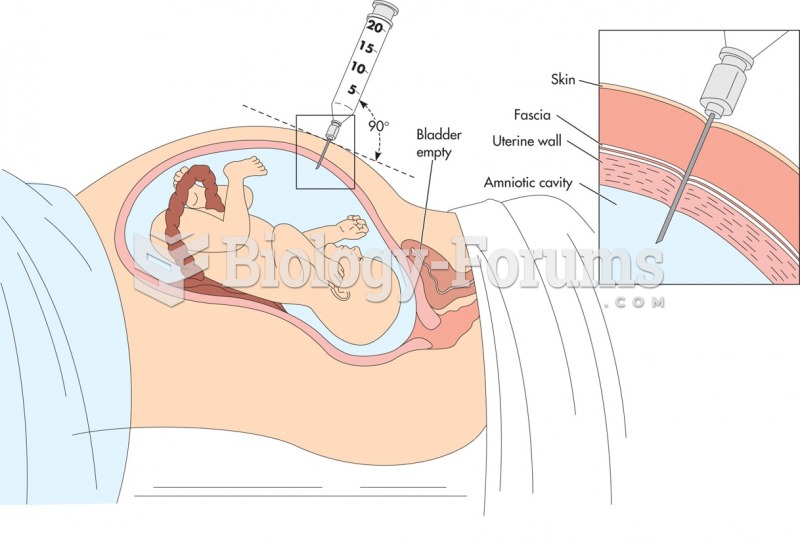
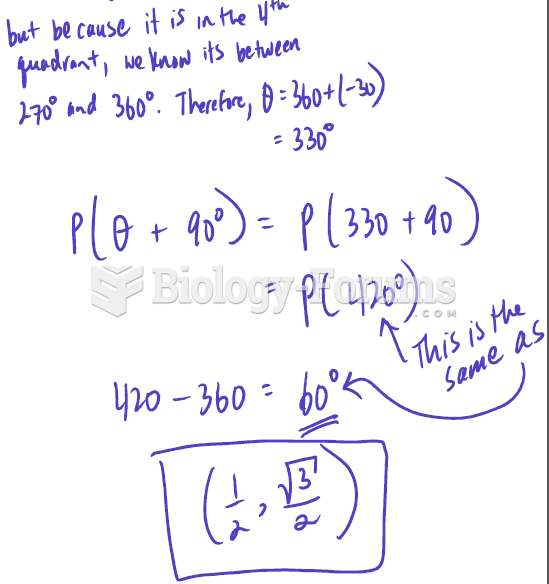
|
|
|
The Romans did not use numerals to indicate fractions but instead used words to indicate parts of a whole.
Asthma occurs in one in 11 children and in one in 12 adults. African Americans and Latinos have a higher risk for developing asthma than other groups.
Warfarin was developed as a consequence of the study of a strange bleeding disorder that suddenly occurred in cattle on the northern prairies of the United States in the early 1900s.
Congestive heart failure is a serious disorder that carries a reduced life expectancy. Heart failure is usually a chronic illness, and it may worsen with infection or other physical stressors.
Cancer has been around as long as humankind, but only in the second half of the twentieth century did the number of cancer cases explode.