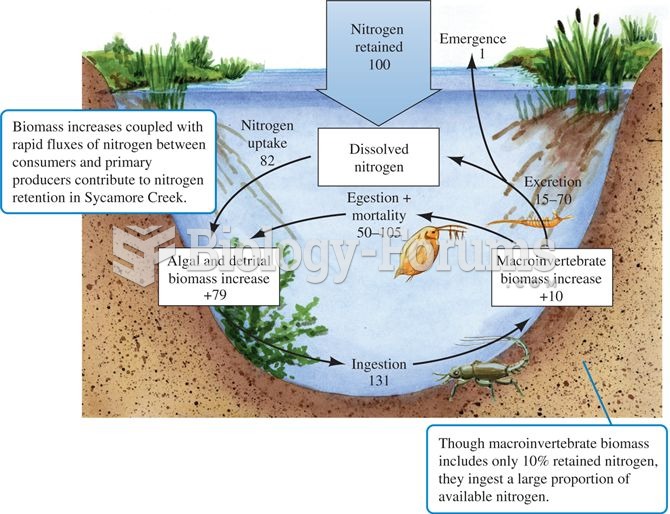
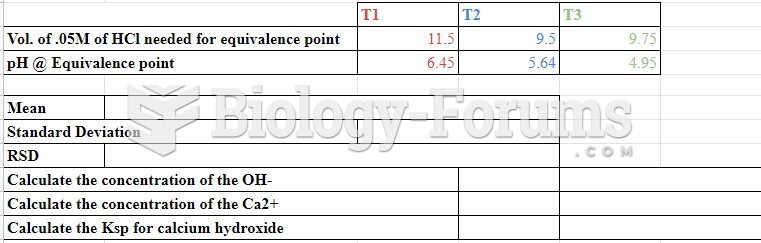
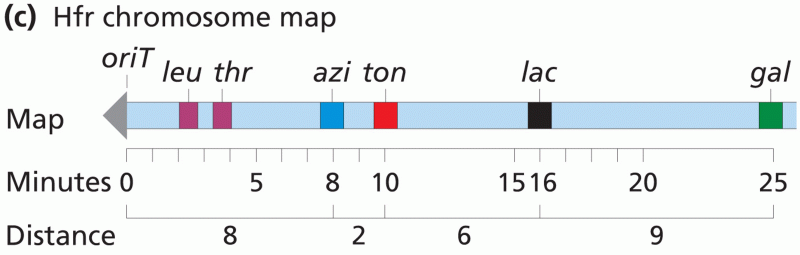
|
|
|
There are over 65,000 known species of protozoa. About 10,000 species are parasitic.
The types of cancer that alpha interferons are used to treat include hairy cell leukemia, melanoma, follicular non-Hodgkin's lymphoma, and AIDS-related Kaposi's sarcoma.
Certain chemicals, after ingestion, can be converted by the body into cyanide. Most of these chemicals have been removed from the market, but some old nail polish remover, solvents, and plastics manufacturing solutions can contain these substances.
Always store hazardous household chemicals in their original containers out of reach of children. These include bleach, paint, strippers and products containing turpentine, garden chemicals, oven cleaners, fondue fuels, nail polish, and nail polish remover.
There are more nerve cells in one human brain than there are stars in the Milky Way.
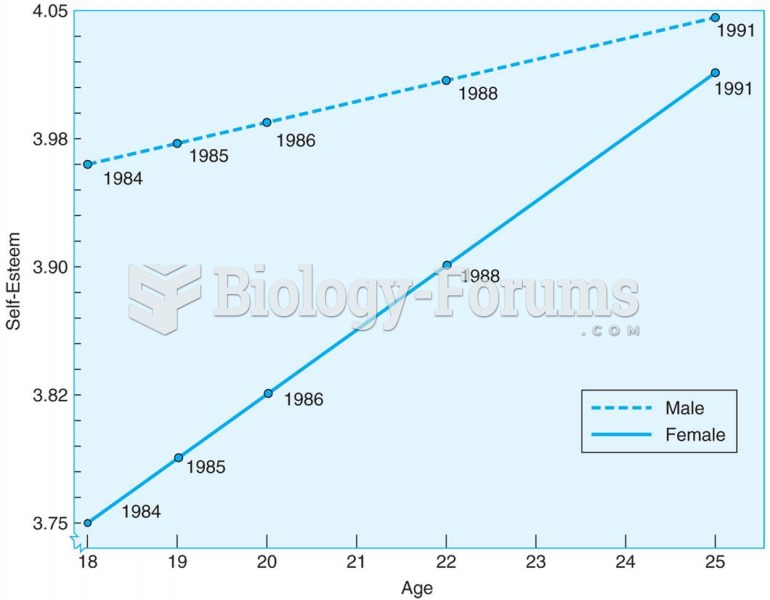 Young adults increase in self-esteem between the ages of 18 and 25, according to this longitudinal s
Young adults increase in self-esteem between the ages of 18 and 25, according to this longitudinal s
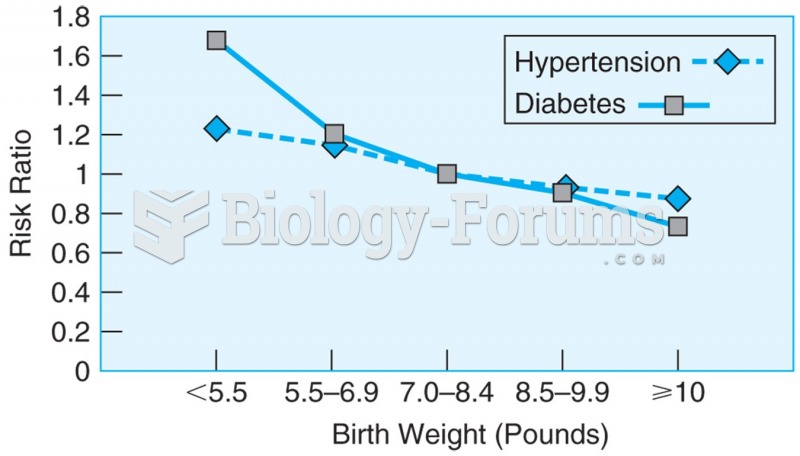 Data from more than 22,000 men over the age of 40, showing the relationship between birth weight and ...
Data from more than 22,000 men over the age of 40, showing the relationship between birth weight and ...