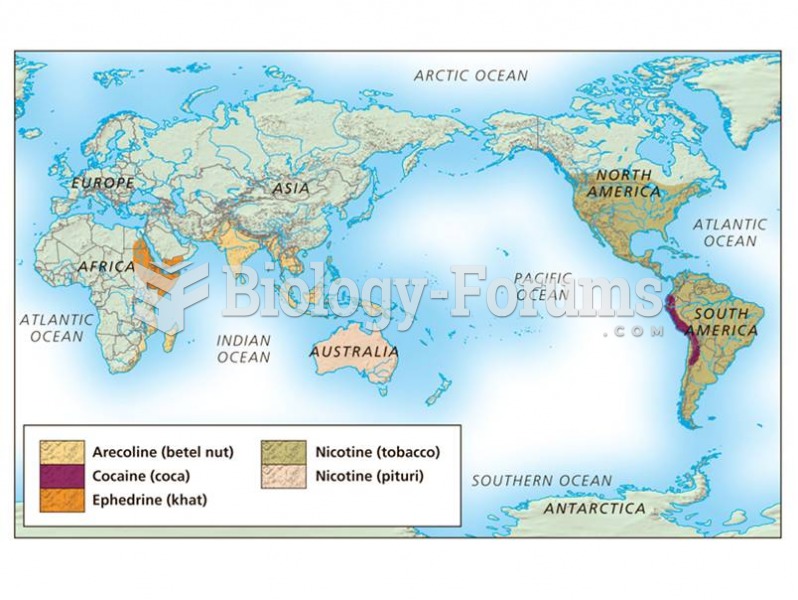
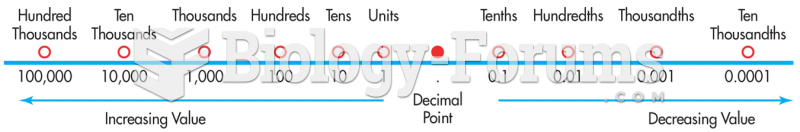
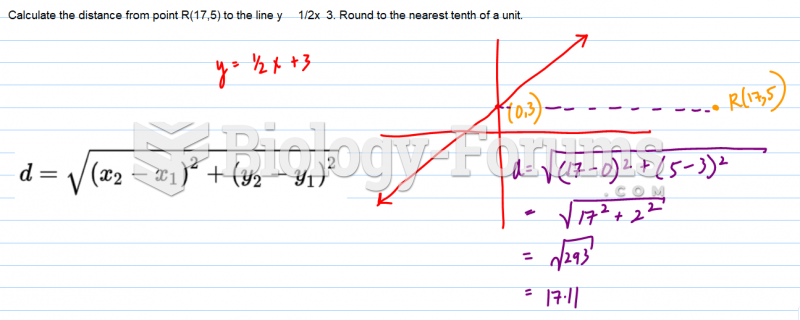
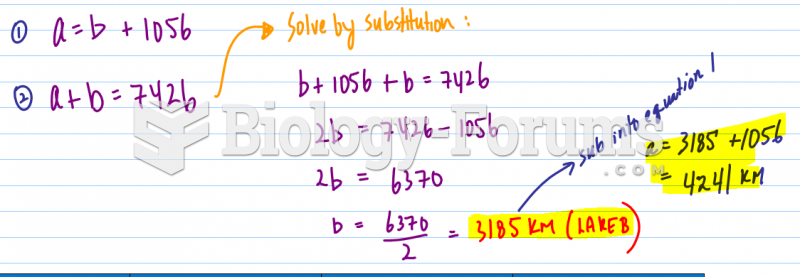
|
|
|
Most childhood vaccines are 90–99% effective in preventing disease. Side effects are rarely serious.
The FDA recognizes 118 routes of administration.
When blood is exposed to air, it clots. Heparin allows the blood to come in direct contact with air without clotting.
As many as 28% of hospitalized patients requiring mechanical ventilators to help them breathe (for more than 48 hours) will develop ventilator-associated pneumonia. Current therapy involves intravenous antibiotics, but new antibiotics that can be inhaled (and more directly treat the infection) are being developed.
To combat osteoporosis, changes in lifestyle and diet are recommended. At-risk patients should include 1,200 to 1,500 mg of calcium daily either via dietary means or with supplements.