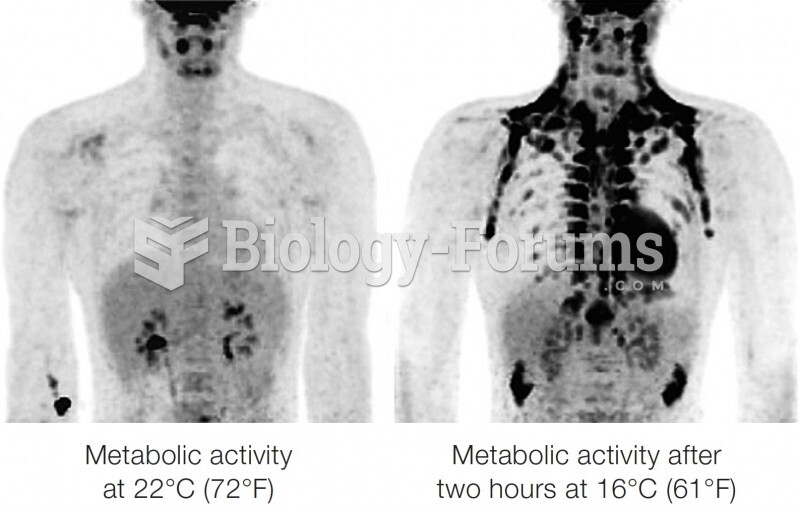
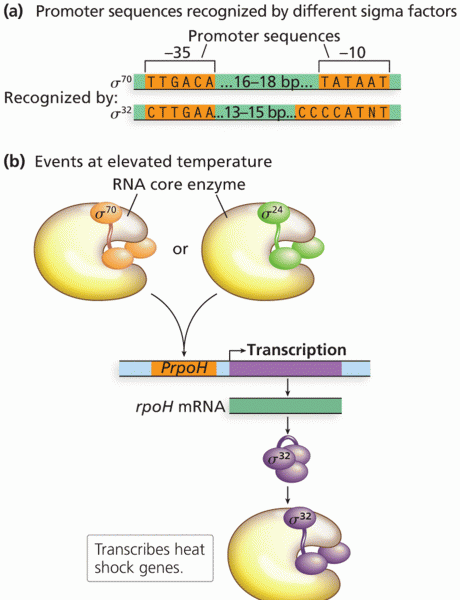
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Adults are resistant to the bacterium that causes Botulism. These bacteria thrive in honey – therefore, honey should never be given to infants since their immune systems are not yet resistant.
Did you know?
There are 20 feet of blood vessels in each square inch of human skin.
Did you know?
Although puberty usually occurs in the early teenage years, the world's youngest parents were two Chinese children who had their first baby when they were 8 and 9 years of age.
Did you know?
Atropine was named after the Greek goddess Atropos, the oldest and ugliest of the three sisters known as the Fates, who controlled the destiny of men.
Did you know?
The B-complex vitamins and vitamin C are not stored in the body and must be replaced each day.