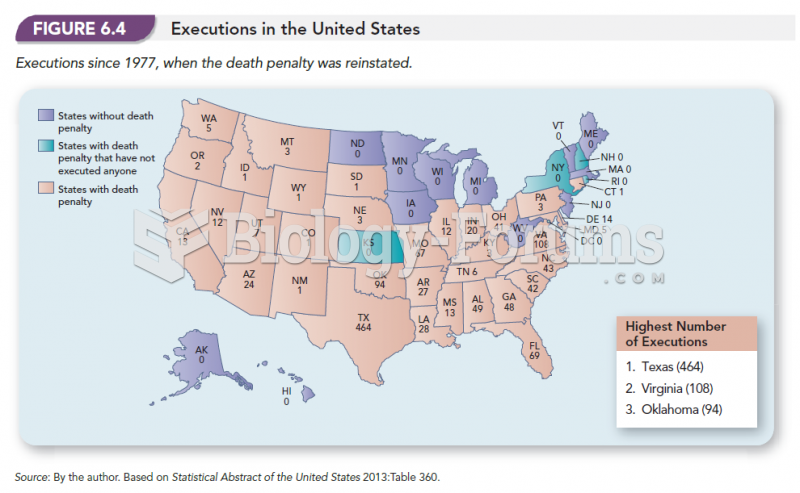
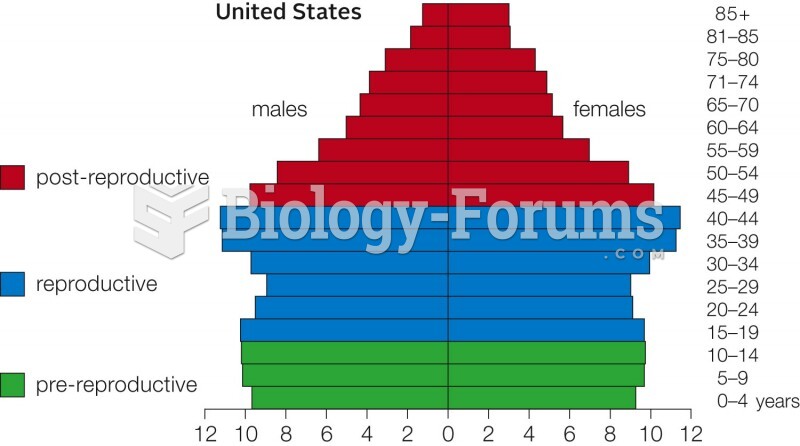
|
|
|
The average older adult in the United States takes five prescription drugs per day. Half of these drugs contain a sedative. Alcohol should therefore be avoided by most senior citizens because of the dangerous interactions between alcohol and sedatives.
Approximately 70% of expectant mothers report experiencing some symptoms of morning sickness during the first trimester of pregnancy.
Approximately 500,000 babies are born each year in the United States to teenage mothers.
More than 20 million Americans cite use of marijuana within the past 30 days, according to the National Survey on Drug Use and Health (NSDUH). More than 8 million admit to using it almost every day.
The Romans did not use numerals to indicate fractions but instead used words to indicate parts of a whole.
 When the United States gets stuck over a controversial issue—usually something a divided Congress ca
When the United States gets stuck over a controversial issue—usually something a divided Congress ca
 Booker T. Washington in his office at Tuskegee Institute, 1900. Washington chose a policy of accommo
Booker T. Washington in his office at Tuskegee Institute, 1900. Washington chose a policy of accommo