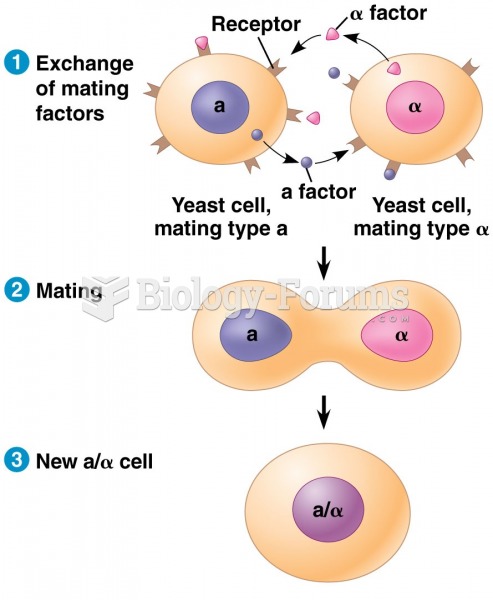
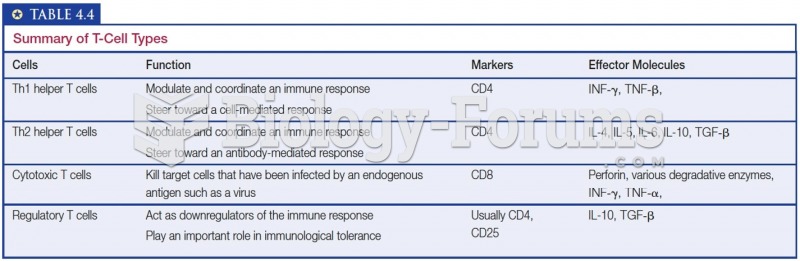
|
|
|
Your skin wrinkles if you stay in the bathtub a long time because the outermost layer of skin (which consists of dead keratin) swells when it absorbs water. It is tightly attached to the skin below it, so it compensates for the increased area by wrinkling. This happens to the hands and feet because they have the thickest layer of dead keratin cells.
Multiple sclerosis is a condition wherein the body's nervous system is weakened by an autoimmune reaction that attacks the myelin sheaths of neurons.
More than 150,000 Americans killed by cardiovascular disease are younger than the age of 65 years.
After 5 years of being diagnosed with rheumatoid arthritis, one every three patients will no longer be able to work.
Malaria was not eliminated in the United States until 1951. The term eliminated means that no new cases arise in a country for 3 years.