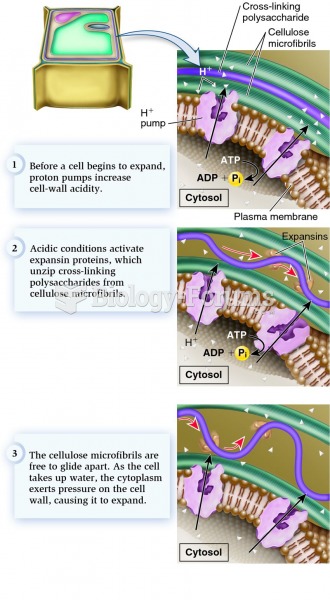
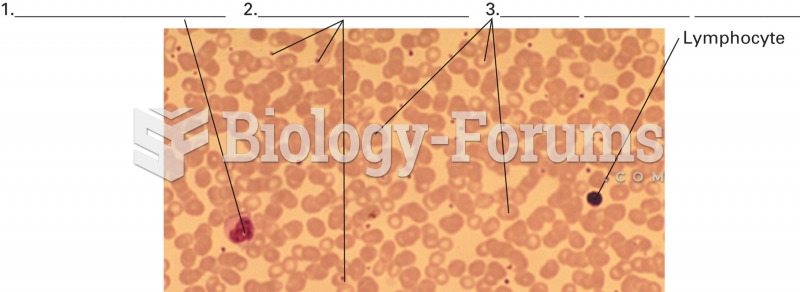
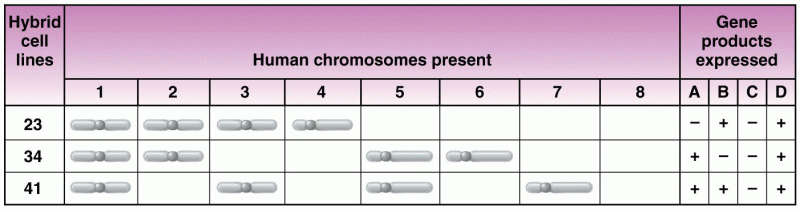
|
|
|
The first-known contraceptive was crocodile dung, used in Egypt in 2000 BC. Condoms were also reportedly used, made of animal bladders or intestines.
More than nineteen million Americans carry the factor V gene that causes blood clots, pulmonary embolism, and heart disease.
Always store hazardous household chemicals in their original containers out of reach of children. These include bleach, paint, strippers and products containing turpentine, garden chemicals, oven cleaners, fondue fuels, nail polish, and nail polish remover.
Elderly adults are living longer, and causes of death are shifting. At the same time, autopsy rates are at or near their lowest in history.
For high blood pressure (hypertension), a new class of drug, called a vasopeptidase blocker (inhibitor), has been developed. It decreases blood pressure by simultaneously dilating the peripheral arteries and increasing the body's loss of salt.