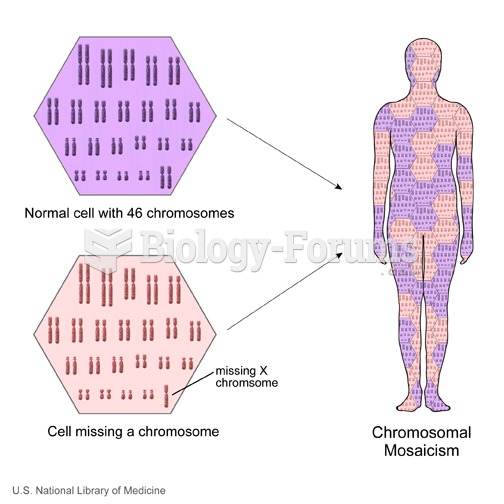
|
|
|
Only 12 hours after an egg cell is fertilized by a sperm cell, the egg cell starts to divide. As it continues to divide, it moves along the fallopian tube toward the uterus at about 1 inch per day.
Chronic marijuana use can damage the white blood cells and reduce the immune system's ability to respond to disease by as much as 40%. Without a strong immune system, the body is vulnerable to all kinds of degenerative and infectious diseases.
The U.S. Pharmacopeia Medication Errors Reporting Program states that approximately 50% of all medication errors involve insulin.
The Centers for Disease Control and Prevention (CDC) was originally known as the Communicable Disease Center, which was formed to fight malaria. It was originally headquartered in Atlanta, Georgia, since the Southern states faced the worst threat from malaria.
If all the neurons in the human body were lined up, they would stretch more than 600 miles.
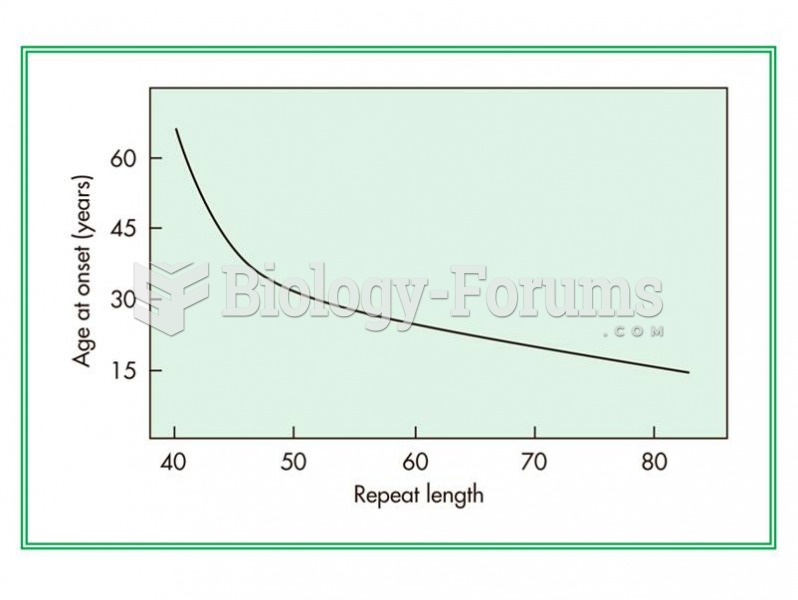 Relationship between the number of CAG repeats in a gene and the age of onset of Huntington disease.
Relationship between the number of CAG repeats in a gene and the age of onset of Huntington disease.
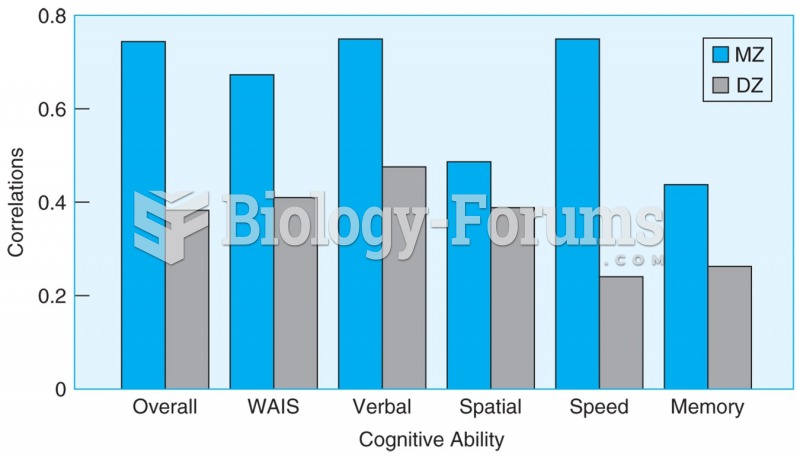 Correlations on tests for a number of cognitive abilities are higher for monozygotic twin pairs (who ...
Correlations on tests for a number of cognitive abilities are higher for monozygotic twin pairs (who ...