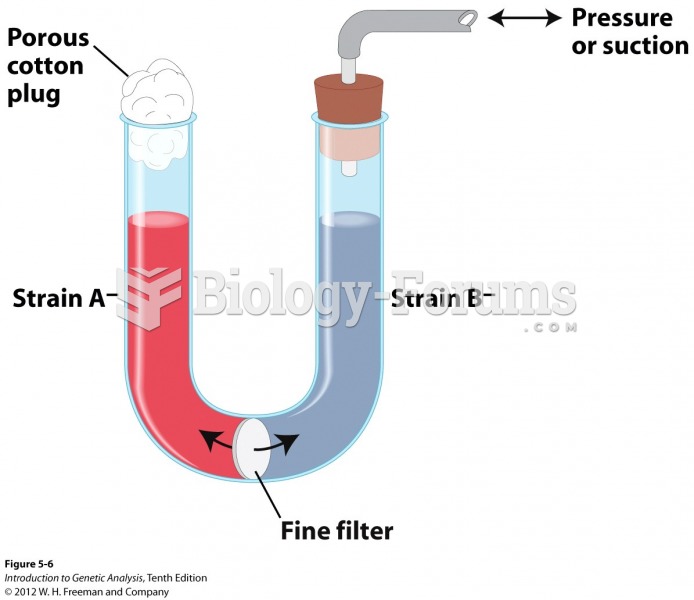
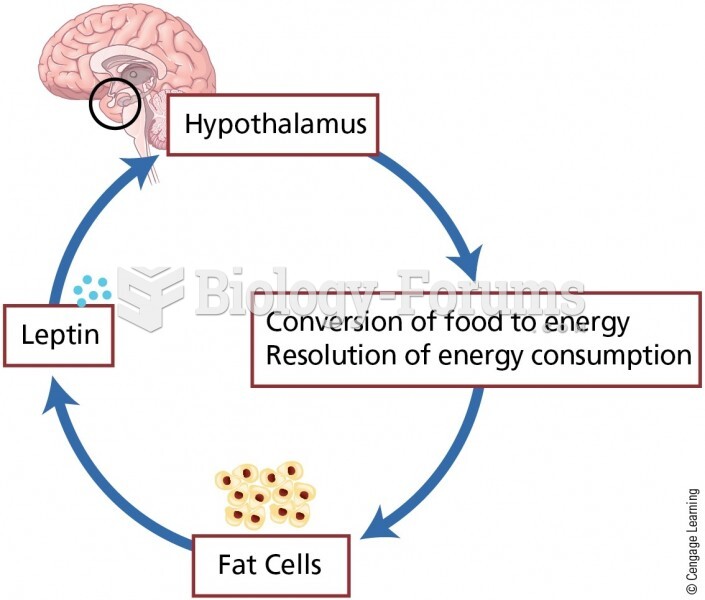
|
|
|
Though newer “smart” infusion pumps are increasingly becoming more sophisticated, they cannot prevent all programming and administration errors. Health care professionals that use smart infusion pumps must still practice the rights of medication administration and have other professionals double-check all high-risk infusions.
There are more sensory neurons in the tongue than in any other part of the body.
There are more bacteria in your mouth than there are people in the world.
In the ancient and medieval periods, dysentery killed about ? of all babies before they reach 12 months of age. The disease was transferred through contaminated drinking water, because there was no way to adequately dispose of sewage, which contaminated the water.
In 1864, the first barbiturate (barbituric acid) was synthesized.