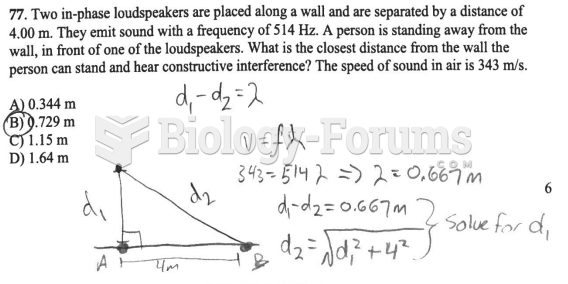
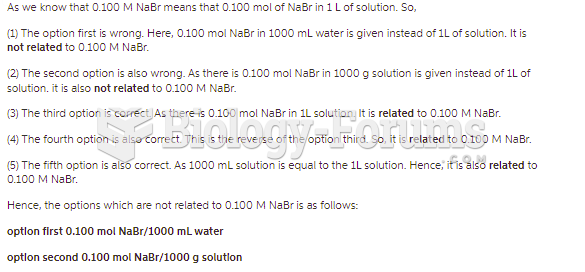
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Asthma cases in Americans are about 75% higher today than they were in 1980.
Did you know?
Eating food that has been cooked with poppy seeds may cause you to fail a drug screening test, because the seeds contain enough opiate alkaloids to register as a positive.
Did you know?
The oldest recorded age was 122. Madame Jeanne Calment was born in France in 1875 and died in 1997. She was a vegetarian and loved olive oil, port wine, and chocolate.
Did you know?
According to the CDC, approximately 31.7% of the U.S. population has high low-density lipoprotein (LDL) or "bad cholesterol" levels.
Did you know?
Individuals are never “cured” of addictions. Instead, they learn how to manage their disease to lead healthy, balanced lives.