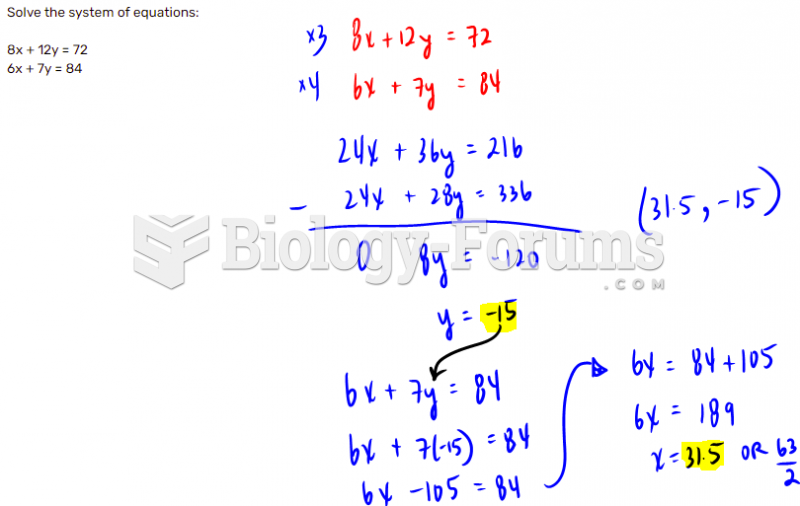
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Since 1988, the CDC has reported a 99% reduction in bacterial meningitis caused by Haemophilus influenzae, due to the introduction of the vaccine against it.
Did you know?
More than one-third of adult Americans are obese. Diseases that kill the largest number of people annually, such as heart disease, cancer, diabetes, stroke, and hypertension, can be attributed to diet.
Did you know?
Calcitonin is a naturally occurring hormone. In women who are at least 5 years beyond menopause, it slows bone loss and increases spinal bone density.
Did you know?
In 1844, Charles Goodyear obtained the first patent for a rubber condom.
Did you know?
Earwax has antimicrobial properties that reduce the viability of bacteria and fungus in the human ear.