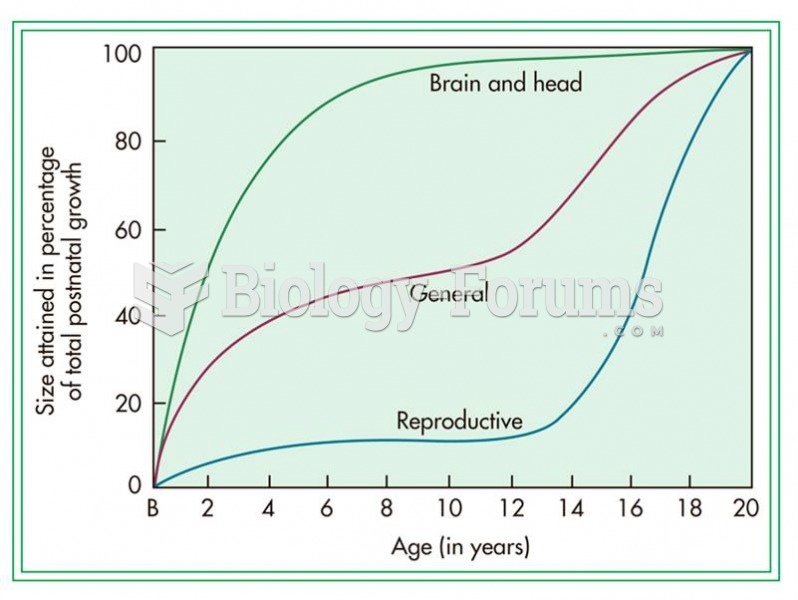
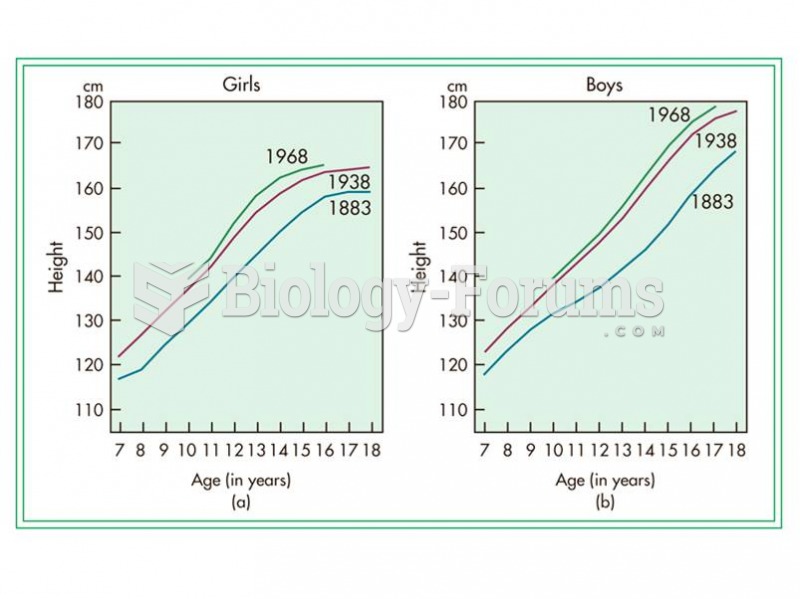
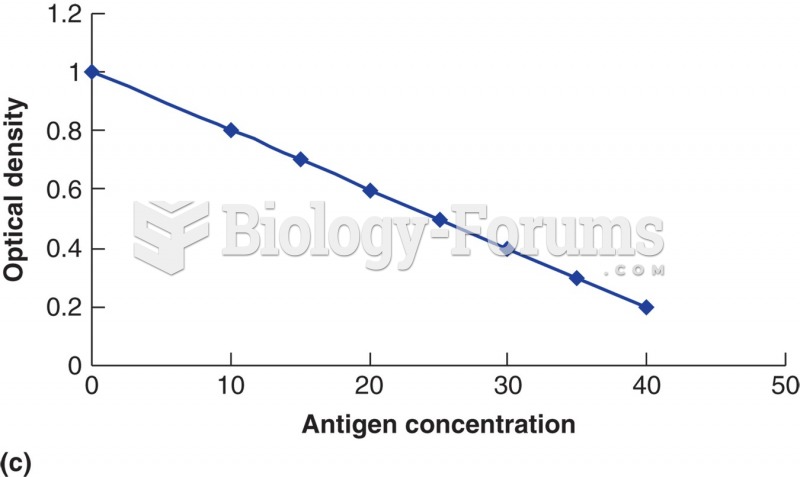
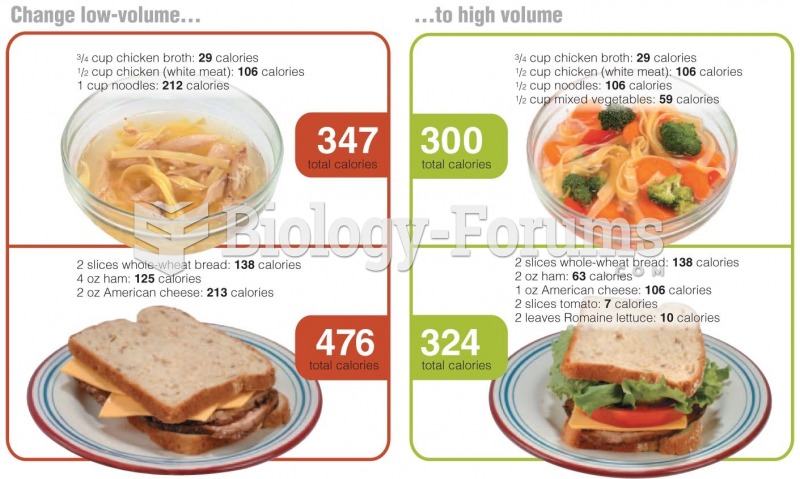
|
|
|
Illicit drug use costs the United States approximately $181 billion every year.
Urine turns bright yellow if larger than normal amounts of certain substances are consumed; one of these substances is asparagus.
As many as 28% of hospitalized patients requiring mechanical ventilators to help them breathe (for more than 48 hours) will develop ventilator-associated pneumonia. Current therapy involves intravenous antibiotics, but new antibiotics that can be inhaled (and more directly treat the infection) are being developed.
Multiple sclerosis is a condition wherein the body's nervous system is weakened by an autoimmune reaction that attacks the myelin sheaths of neurons.
People with high total cholesterol have about two times the risk for heart disease as people with ideal levels.