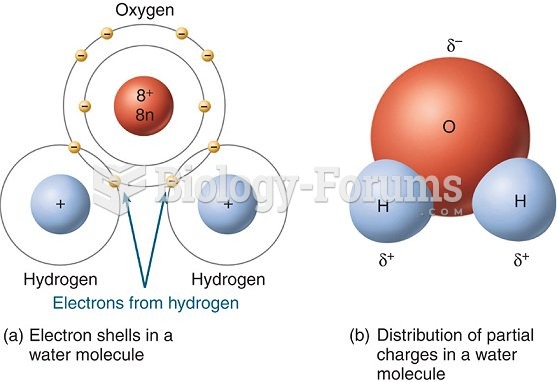
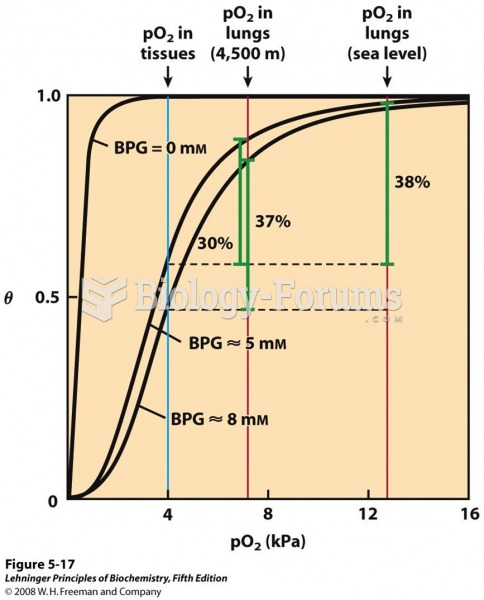
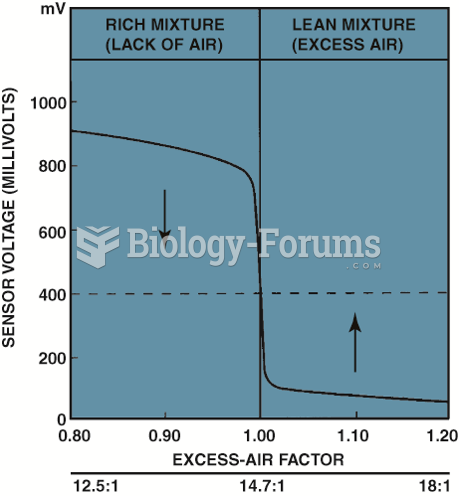
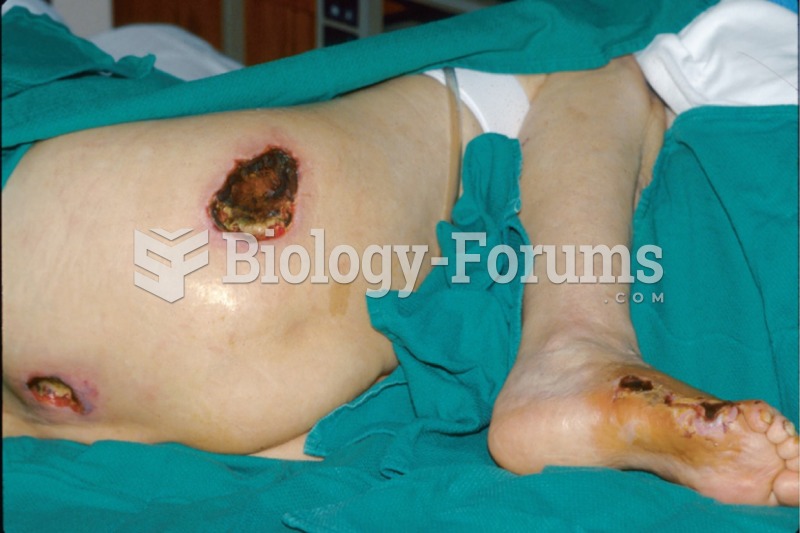
|
|
|
In women, pharmacodynamic differences include increased sensitivity to (and increased effectiveness of) beta-blockers, opioids, selective serotonin reuptake inhibitors, and typical antipsychotics.
Intradermal injections are somewhat difficult to correctly administer because the skin layers are so thin that it is easy to accidentally punch through to the deeper subcutaneous layer.
Congestive heart failure is a serious disorder that carries a reduced life expectancy. Heart failure is usually a chronic illness, and it may worsen with infection or other physical stressors.
Every 10 seconds, a person in the United States goes to the emergency room complaining of head pain. About 1.2 million visits are for acute migraine attacks.
About 60% of newborn infants in the United States are jaundiced; that is, they look yellow. Kernicterus is a form of brain damage caused by excessive jaundice. When babies begin to be affected by excessive jaundice and begin to have brain damage, they become excessively lethargic.