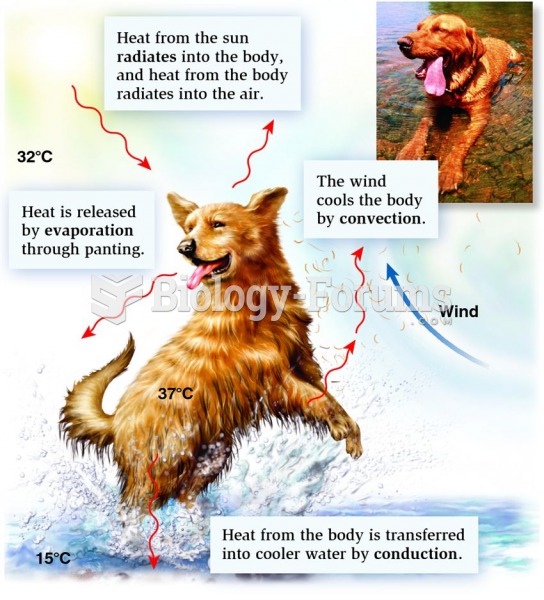
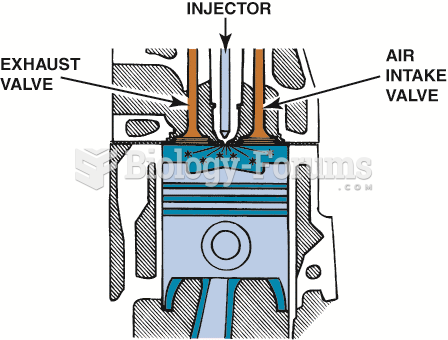
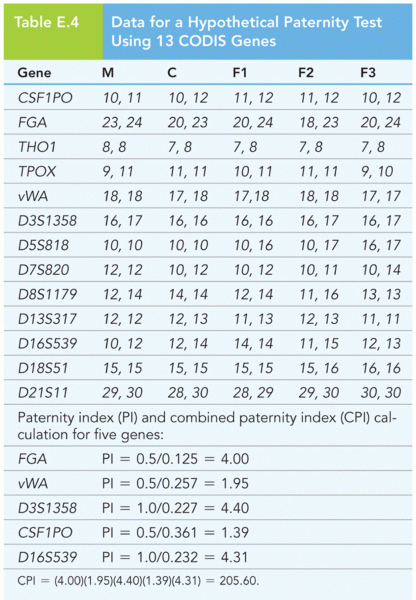
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
There are approximately 3 million unintended pregnancies in the United States each year.
Did you know?
There are over 65,000 known species of protozoa. About 10,000 species are parasitic.
Did you know?
In Eastern Europe and Russia, interferon is administered intranasally in varied doses for the common cold and influenza. It is claimed that this treatment can lower the risk of infection by as much as 60–70%.
Did you know?
Women are two-thirds more likely than men to develop irritable bowel syndrome. This may be attributable to hormonal changes related to their menstrual cycles.
Did you know?
The effects of organophosphate poisoning are referred to by using the abbreviations “SLUD” or “SLUDGE,” It stands for: salivation, lacrimation, urination, defecation, GI upset, and emesis.