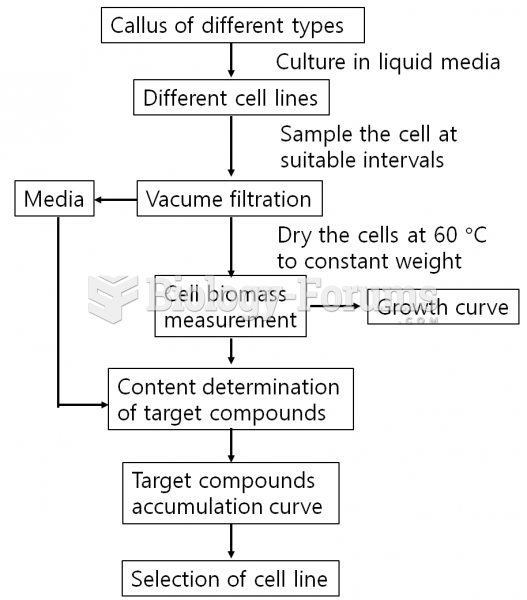
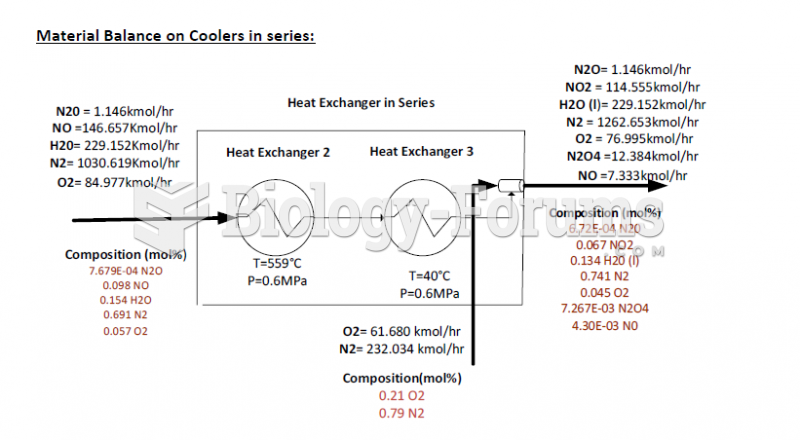
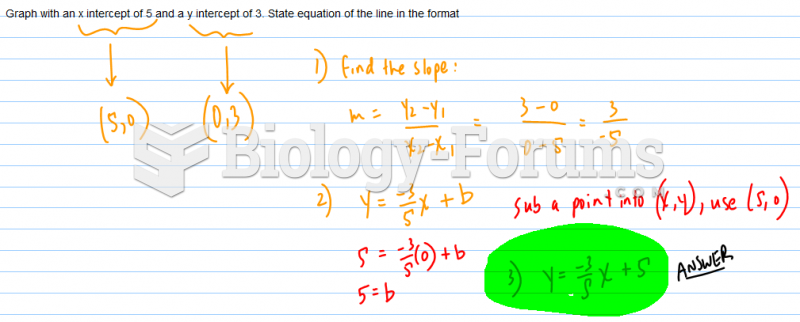
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Recent studies have shown that the number of medication errors increases in relation to the number of orders that are verified per pharmacist, per work shift.
Did you know?
It is important to read food labels and choose foods with low cholesterol and saturated trans fat. You should limit saturated fat to no higher than 6% of daily calories.
Did you know?
The first oral chemotherapy drug for colon cancer was approved by FDA in 2001.
Did you know?
About 100 new prescription or over-the-counter drugs come into the U.S. market every year.
Did you know?
Immunoglobulin injections may give short-term protection against, or reduce severity of certain diseases. They help people who have an inherited problem making their own antibodies, or those who are having certain types of cancer treatments.