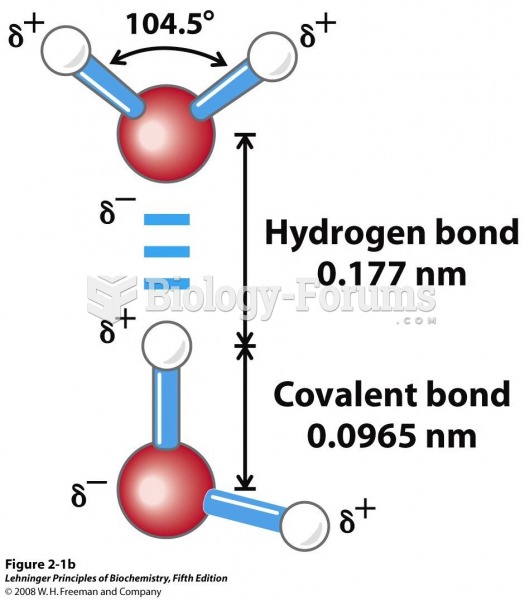
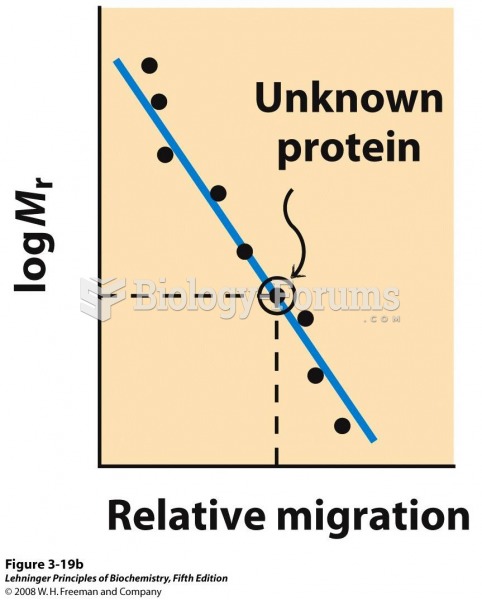
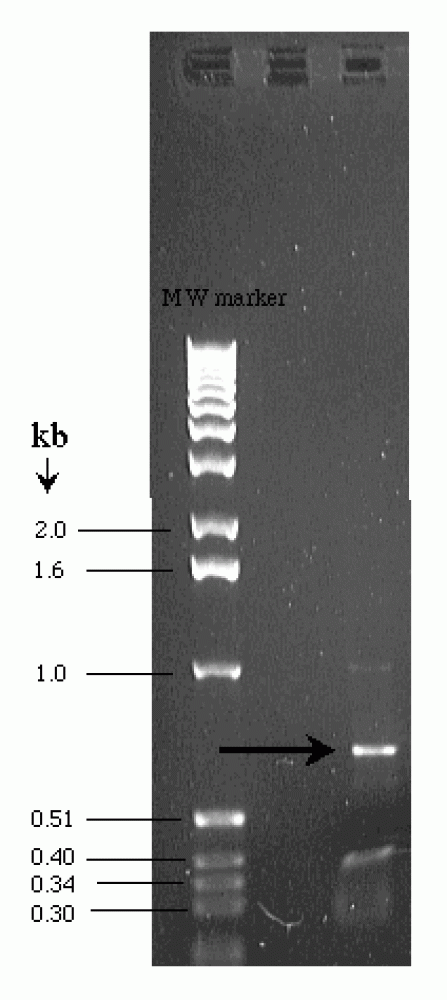
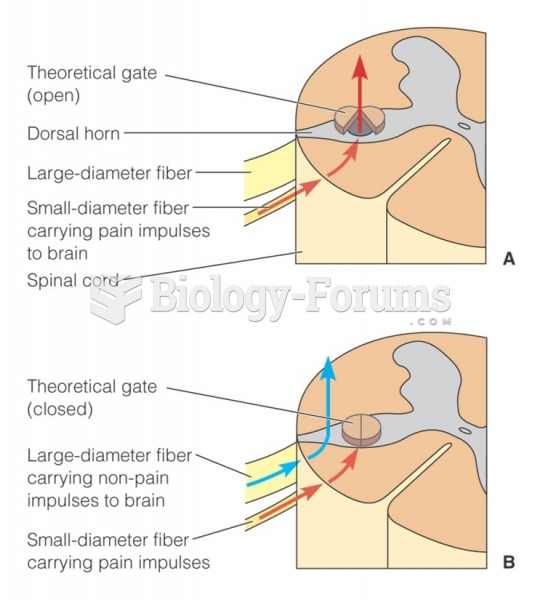
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
The calories found in one piece of cherry cheesecake could light a 60-watt light bulb for 1.5 hours.
Did you know?
A seasonal flu vaccine is the best way to reduce the chances you will get seasonal influenza and spread it to others.
Did you know?
There are approximately 3 million unintended pregnancies in the United States each year.
Did you know?
There are more sensory neurons in the tongue than in any other part of the body.
Did you know?
About one in five American adults and teenagers have had a genital herpes infection—and most of them don't know it. People with genital herpes have at least twice the risk of becoming infected with HIV if exposed to it than those people who do not have genital herpes.