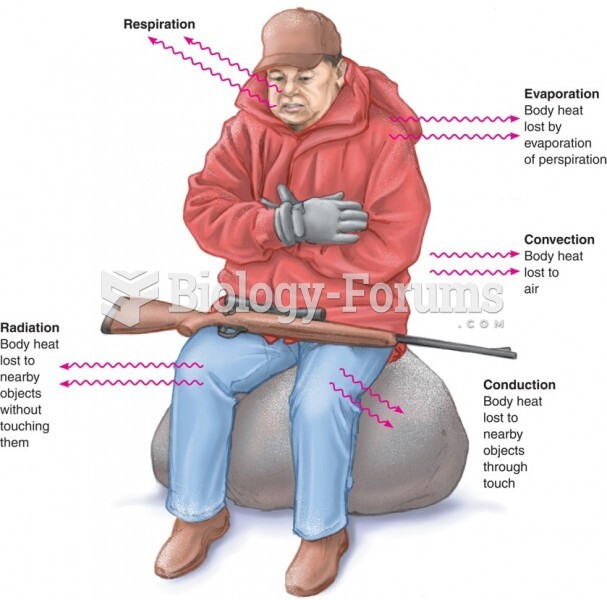
|
|
|
On average, someone in the United States has a stroke about every 40 seconds. This is about 795,000 people per year.
Dogs have been used in studies to detect various cancers in human subjects. They have been trained to sniff breath samples from humans that were collected by having them breathe into special tubes. These people included 55 lung cancer patients, 31 breast cancer patients, and 83 cancer-free patients. The dogs detected 54 of the 55 lung cancer patients as having cancer, detected 28 of the 31 breast cancer patients, and gave only three false-positive results (detecting cancer in people who didn't have it).
More than 50% of American adults have oral herpes, which is commonly known as "cold sores" or "fever blisters." The herpes virus can be active on the skin surface without showing any signs or causing any symptoms.
In the ancient and medieval periods, dysentery killed about ? of all babies before they reach 12 months of age. The disease was transferred through contaminated drinking water, because there was no way to adequately dispose of sewage, which contaminated the water.
In ancient Rome, many of the richer people in the population had lead-induced gout. The reason for this is unclear. Lead poisoning has also been linked to madness.
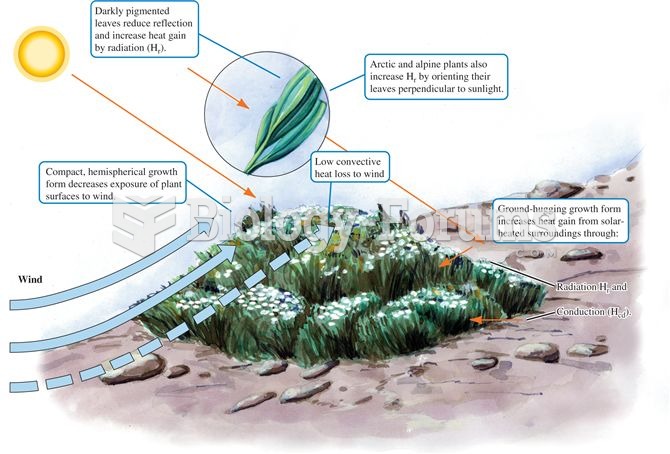 Arctic and alpine cushion plant form and orientation increases heat gain from sunlight and the surro
Arctic and alpine cushion plant form and orientation increases heat gain from sunlight and the surro
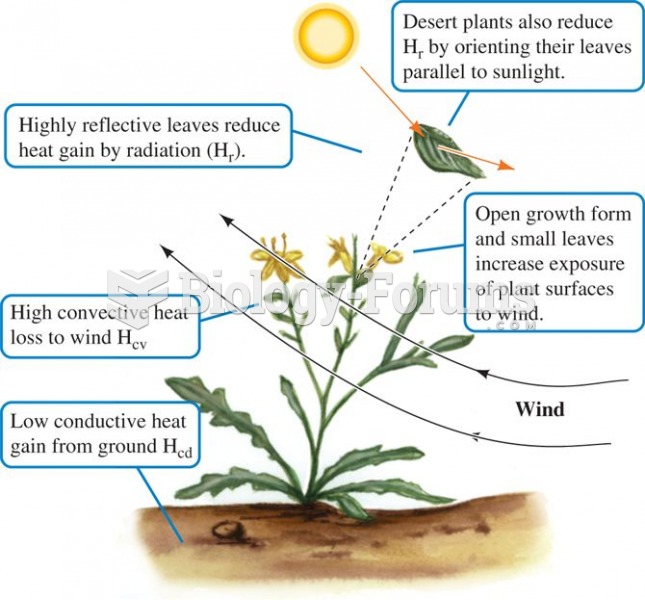 The form and orientation of desert plants reduces heat gain from the environment and facilitates coo
The form and orientation of desert plants reduces heat gain from the environment and facilitates coo
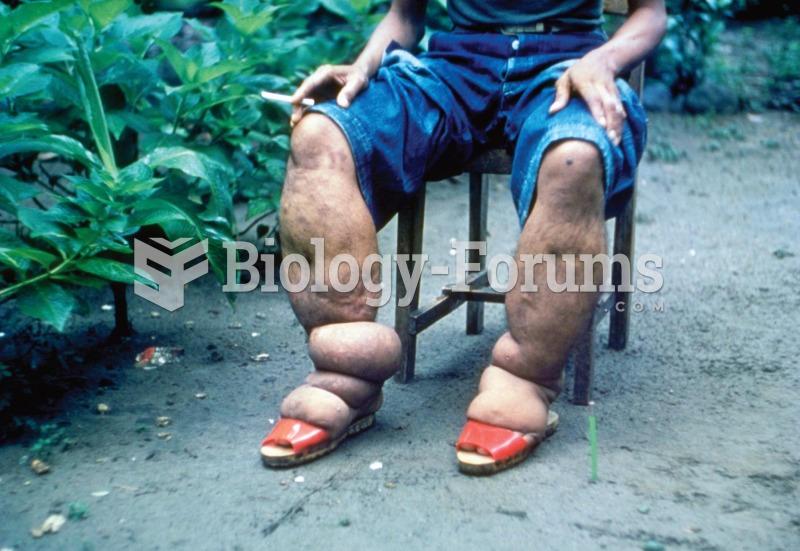 Inflammation. Inflammation is characterized by the presence of swelling, redness, heat, and pain. Sw
Inflammation. Inflammation is characterized by the presence of swelling, redness, heat, and pain. Sw
 Apply the heat source, and ask for immediate feedback on the heat intensity felt by the recipient. ...
Apply the heat source, and ask for immediate feedback on the heat intensity felt by the recipient. ...