|
|
|
Asthma cases in Americans are about 75% higher today than they were in 1980.
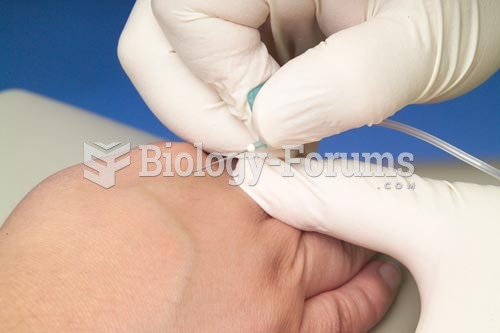
When blood is exposed to air, it clots. Heparin allows the blood to come in direct contact with air without clotting.
Eat fiber! A diet high in fiber can help lower cholesterol levels by as much as 10%.
Many people have small pouches in their colons that bulge outward through weak spots. Each pouch is called a diverticulum. About 10% of Americans older than age 40 years have diverticulosis, which, when the pouches become infected or inflamed, is called diverticulitis. The main cause of diverticular disease is a low-fiber diet.
Allergies play a major part in the health of children. The most prevalent childhood allergies are milk, egg, soy, wheat, peanuts, tree nuts, and seafood.