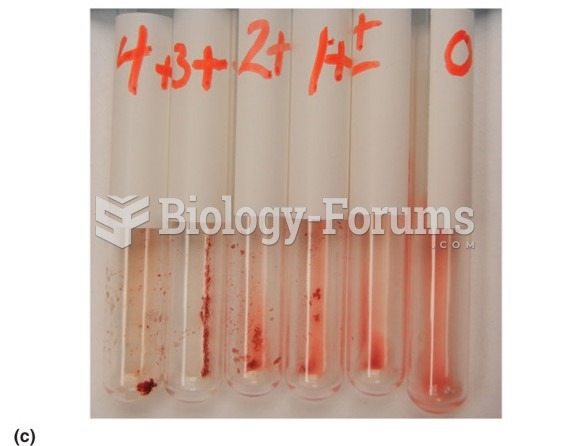
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
There are 60,000 miles of blood vessels in every adult human.
Did you know?
The U.S. Preventive Services Task Force recommends that all women age 65 years of age or older should be screened with bone densitometry.
Did you know?
People with high total cholesterol have about two times the risk for heart disease as people with ideal levels.
Did you know?
During the twentieth century, a variant of the metric system was used in Russia and France in which the base unit of mass was the tonne. Instead of kilograms, this system used millitonnes (mt).
Did you know?
Atropine, along with scopolamine and hyoscyamine, is found in the Datura stramonium plant, which gives hallucinogenic effects and is also known as locoweed.