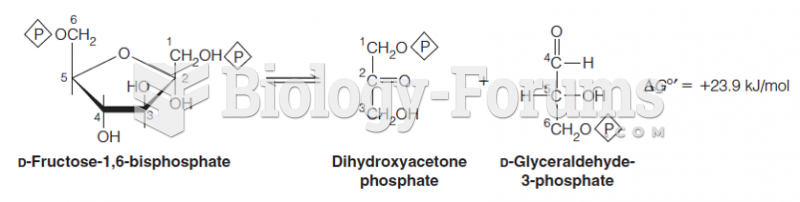
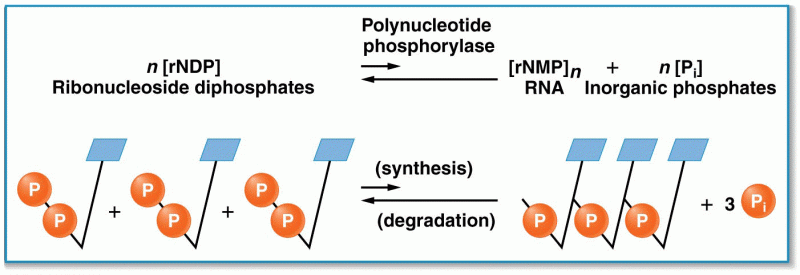
|
|
|
More than 34,000 trademarked medication names and more than 10,000 generic medication names are in use in the United States.
The first monoclonal antibodies were made exclusively from mouse cells. Some are now fully human, which means they are likely to be safer and may be more effective than older monoclonal antibodies.
Women are two-thirds more likely than men to develop irritable bowel syndrome. This may be attributable to hormonal changes related to their menstrual cycles.
All patients with hyperparathyroidism will develop osteoporosis. The parathyroid glands maintain blood calcium within the normal range. All patients with this disease will continue to lose calcium from their bones every day, and there is no way to prevent the development of osteoporosis as a result.
Famous people who died from poisoning or drug overdose include, Adolf Hitler, Socrates, Juan Ponce de Leon, Marilyn Monroe, Judy Garland, and John Belushi.