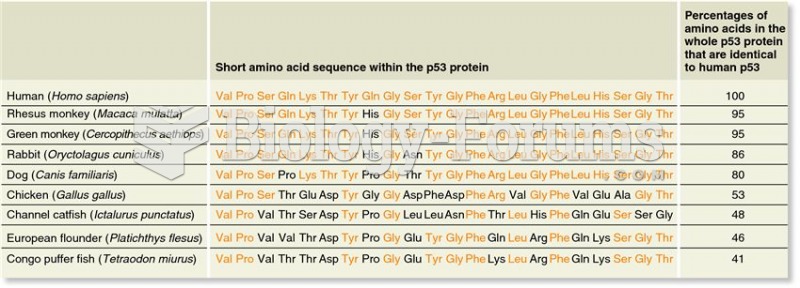
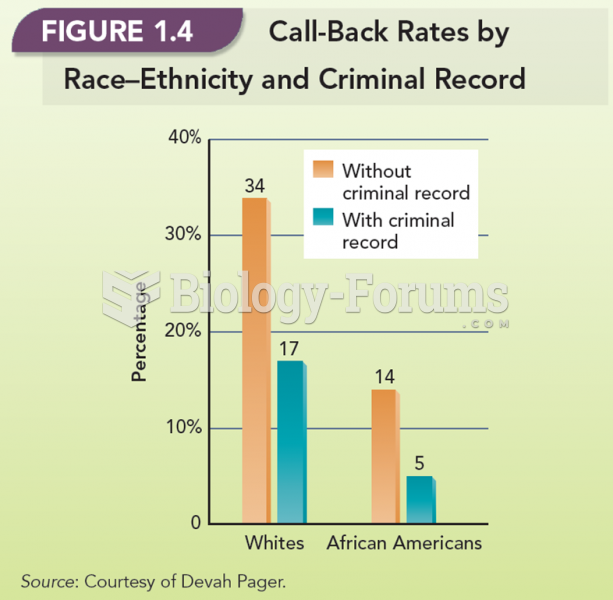
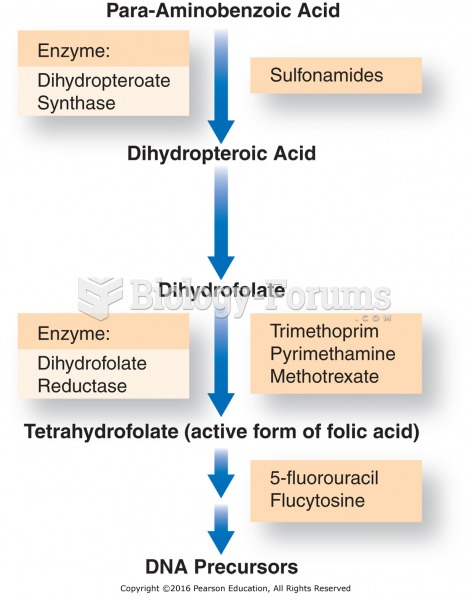
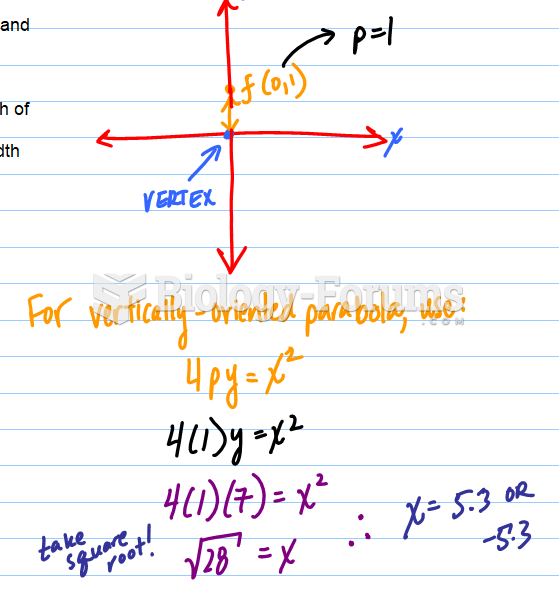
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Asthma attacks and symptoms usually get started by specific triggers (such as viruses, allergies, gases, and air particles). You should talk to your doctor about these triggers and find ways to avoid or get rid of them.
Did you know?
The average adult has about 21 square feet of skin.
Did you know?
The people with the highest levels of LDL are Mexican American males and non-Hispanic black females.
Did you know?
Approximately 15–25% of recognized pregnancies end in miscarriage. However, many miscarriages often occur before a woman even knows she is pregnant.
Did you know?
Patients who cannot swallow may receive nutrition via a parenteral route—usually, a catheter is inserted through the chest into a large vein going into the heart.