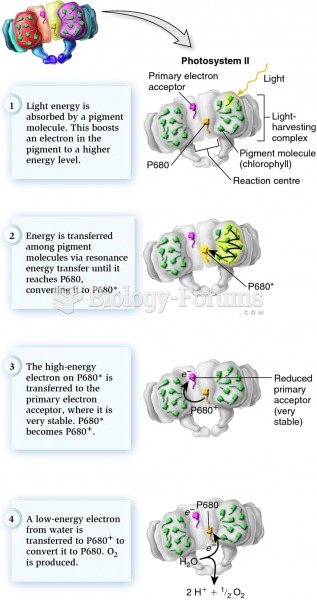
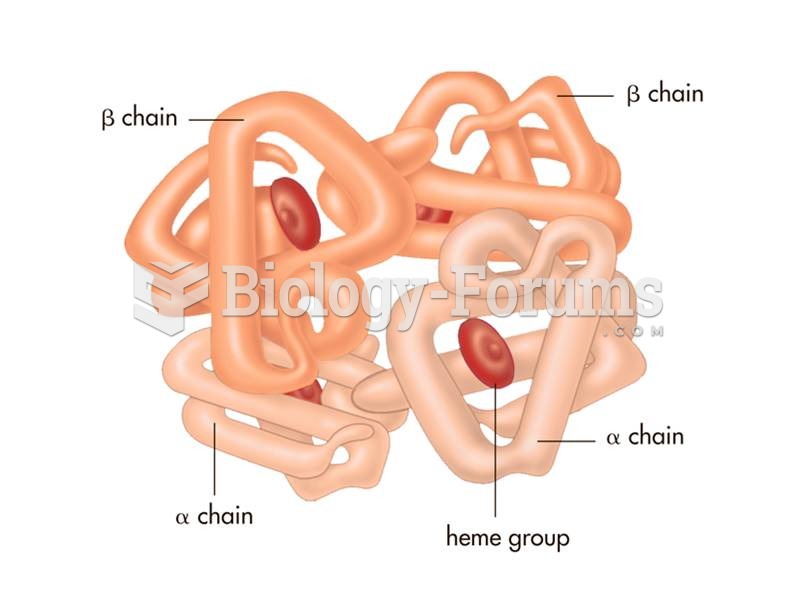
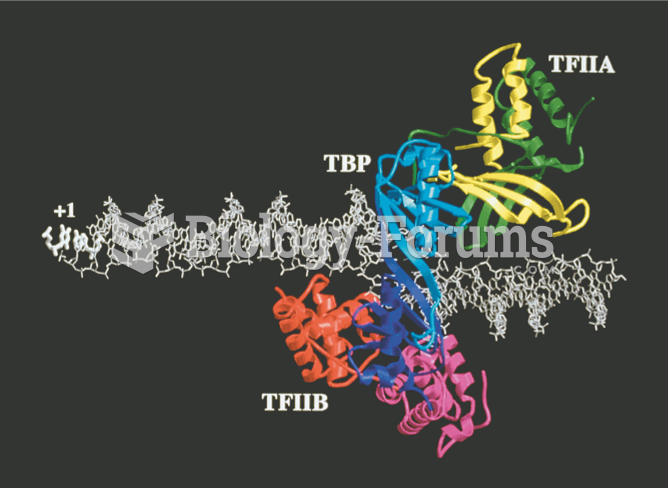
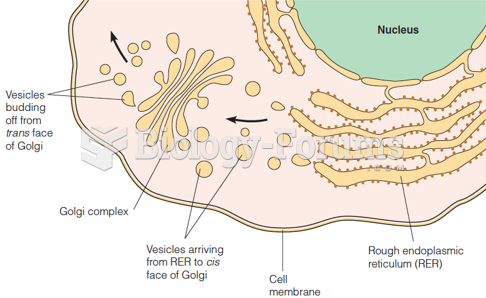
|
|
|
After a vasectomy, it takes about 12 ejaculations to clear out sperm that were already beyond the blocked area.
Although puberty usually occurs in the early teenage years, the world's youngest parents were two Chinese children who had their first baby when they were 8 and 9 years of age.
About one in five American adults and teenagers have had a genital herpes infection—and most of them don't know it. People with genital herpes have at least twice the risk of becoming infected with HIV if exposed to it than those people who do not have genital herpes.
The newest statin drug, rosuvastatin, has been called a superstatin because it appears to reduce LDL cholesterol to a greater degree than the other approved statin drugs.
In most cases, kidneys can recover from almost complete loss of function, such as in acute kidney (renal) failure.