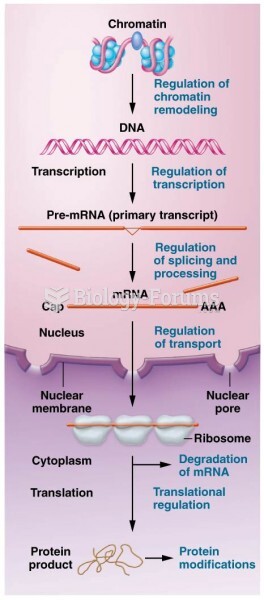
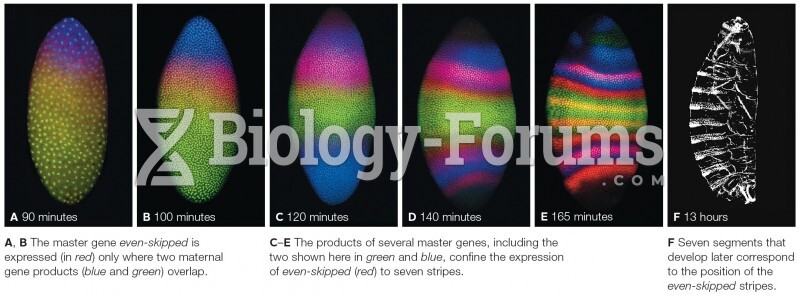
|
|
|
Women are 50% to 75% more likely than men to experience an adverse drug reaction.
In most cases, kidneys can recover from almost complete loss of function, such as in acute kidney (renal) failure.
The B-complex vitamins and vitamin C are not stored in the body and must be replaced each day.
A cataract is a clouding of the eyes' natural lens. As we age, some clouding of the lens may occur. The first sign of a cataract is usually blurry vision. Although glasses and other visual aids may at first help a person with cataracts, surgery may become inevitable. Cataract surgery is very successful in restoring vision, and it is the most frequently performed surgery in the United States.
Patients who have undergone chemotherapy for the treatment of cancer often complain of a lack of mental focus; memory loss; and a general diminution in abilities such as multitasking, attention span, and general mental agility.