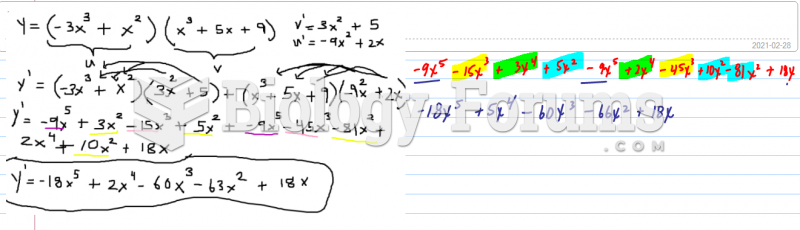
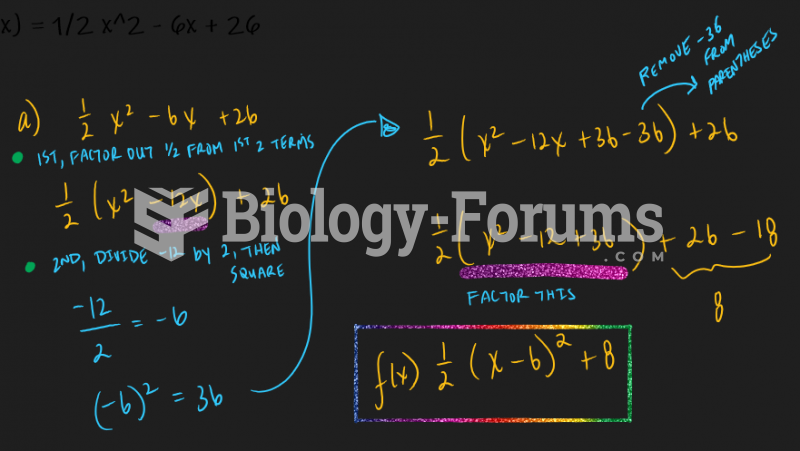
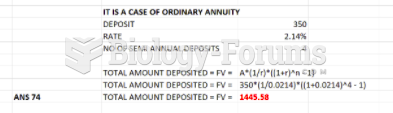
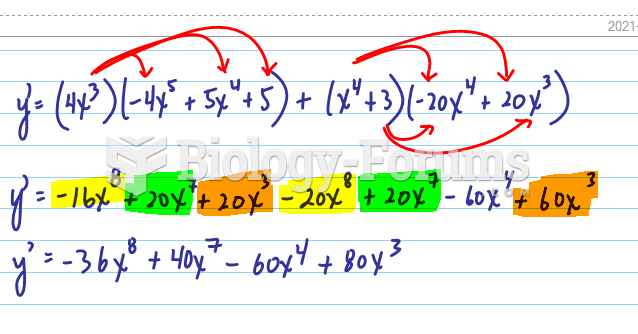This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
The average office desk has 400 times more bacteria on it than a toilet.
Did you know?
More than 4.4billion prescriptions were dispensed within the United States in 2016.
Did you know?
Alcohol acts as a diuretic. Eight ounces of water is needed to metabolize just 1 ounce of alcohol.
Did you know?
Barbituric acid, the base material of barbiturates, was first synthesized in 1863 by Adolph von Bayer. His company later went on to synthesize aspirin for the first time, and Bayer aspirin is still a popular brand today.
Did you know?
Less than one of every three adults with high LDL cholesterol has the condition under control. Only 48.1% with the condition are being treated for it.